The First Yarrowia lipolytica Yeast Models Expressing Hepatitis B Virus X Protein: Changes in Mitochondrial Morphology and Functions
- PMID: 36144419
- PMCID: PMC9501646
- DOI: 10.3390/microorganisms10091817
The First Yarrowia lipolytica Yeast Models Expressing Hepatitis B Virus X Protein: Changes in Mitochondrial Morphology and Functions
Abstract
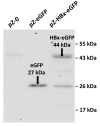
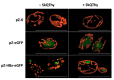
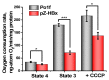
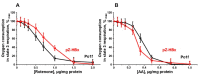
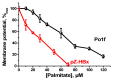
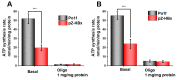
Chronic hepatitis B virus infection is the dominant cause of hepatocellular carcinoma, the main cause of cancer death. HBx protein, a multifunctional protein, is essential for pathogenesis development; however, the underlying mechanisms are not fully understood. The complexity of the system itself, and the intricate interplay of many factors make it difficult to advance in understanding the mechanisms underlying these processes. The most obvious solution is to use simpler systems by reducing the number of interacting factors. Yeast cells are particularly suitable for studying the relationships between oxidative stress, mitochondrial dynamics (mitochondrial fusion and fragmentation), and mitochondrial dysfunction involved in HBx-mediated pathogenesis. For the first time, genetically modified yeast, Y. lipolytica, was created, expressing the hepatitis B virus core protein HBx, as well as a variant fused with eGFP at the C-end. It was found that cells expressing HBx experienced stronger oxidative stress than the control cells. Oxidative stress was alleviated by preincubation with the mitochondria-targeted antioxidant SkQThy. Consistent with these data, in contrast to the control cells (pZ-0) containing numerous mitochondrial forming a mitochondrial reticulum, in cells expressing HBx protein, mitochondria were fragmented, and preincubation with SkQThy partially restored the mitochondrial reticulum. Expression of HBx had a significant influence on the bioenergetic function of mitochondria, making them loosely coupled with decreased respiratory rate and reduced ATP formation. In sum, the first highly promising yeast model for studying the impact of HBx on bioenergy, redox-state, and dynamics of mitochondria in the cell and cross-talk between these parameters was offered. This fairly simple model can be used as a platform for rapid screening of potential therapeutic agents, mitigating the harmful effects of HBx.
Keywords: HBx; Yarrowia lipolytica; hepatitis B virus; hepatocellular carcinoma; heterologous expression; mitochondria-targeted antioxidants; mitochondrial dysfunction; oxidative stress; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Altered Mitochondrial Morphology and Bioenergetics in a New Yeast Model Expressing Aβ42.Int J Mol Sci. 2023 Jan 4;24(2):900. doi: 10.3390/ijms24020900. Int J Mol Sci. 2023. PMID: 36674415 Free PMC article.
-
Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential.J Biol Chem. 2003 Jun 13;278(24):22071-8. doi: 10.1074/jbc.M301606200. Epub 2003 Apr 3. J Biol Chem. 2003. PMID: 12676947
-
Effect of HBx on inflammation and mitochondrial oxidative stress in mouse hepatocytes.Oncol Lett. 2020 Apr;19(4):2861-2869. doi: 10.3892/ol.2020.11404. Epub 2020 Feb 17. Oncol Lett. 2020. PMID: 32218840 Free PMC article.
-
Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma.World J Gastroenterol. 2014 Aug 14;20(30):10238-48. doi: 10.3748/wjg.v20.i30.10238. World J Gastroenterol. 2014. PMID: 25132741 Free PMC article. Review.
-
Hepatitis B x (HBx) as a Component of a Functional Cure for Chronic Hepatitis B.Biomedicines. 2022 Sep 7;10(9):2210. doi: 10.3390/biomedicines10092210. Biomedicines. 2022. PMID: 36140311 Free PMC article. Review.
Cited by
-
Altered Mitochondrial Morphology and Bioenergetics in a New Yeast Model Expressing Aβ42.Int J Mol Sci. 2023 Jan 4;24(2):900. doi: 10.3390/ijms24020900. Int J Mol Sci. 2023. PMID: 36674415 Free PMC article.
References
-
- Chen Z., Yu W., Zhou Q., Zhang J., Jiang H., Hao D., Wang J., Zhou Z., He C., Xiao Z. A Novel lncRNA IHS Promotes Tumor Proliferation and Metastasis in HCC by Regulating the ERK- and AKT/GSK-3β-Signaling Pathways. Mol. Ther. Nucleic Acids. 2019;16:707–720. doi: 10.1016/j.omtn.2019.04.021. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

