Mycobacterium tuberculosis whiB3 and Lipid Metabolism Genes Are Regulated by Host Induced Oxidative Stress
- PMID: 36144423
- PMCID: PMC9506551
- DOI: 10.3390/microorganisms10091821
Mycobacterium tuberculosis whiB3 and Lipid Metabolism Genes Are Regulated by Host Induced Oxidative Stress
Abstract
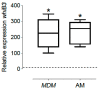
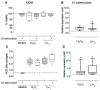
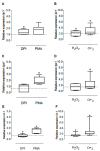
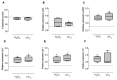
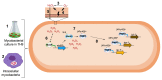
The physiological state of the human macrophage may impact the metabolism and the persistence of Mycobacterium tuberculosis. This pathogen senses and counters the levels of O2, CO, reactive oxygen species (ROS), and pH in macrophages. M. tuberculosis responds to oxidative stress through WhiB3. The goal was to determine the effect of NADPH oxidase (NOX) modulation and oxidative agents on the expression of whiB3 and genes involved in lipid metabolism (lip-Y, Icl-1, and tgs-1) in intracellular mycobacteria. Human macrophages were first treated with NOX modulators such as DPI (ROS inhibitor) and PMA (ROS activator), or with oxidative agents (H2O2 and generator system O2•-), and then infected with mycobacteria. We determined ROS production, cell viability, and expression of whiB3, as well as genes involved in lipid metabolism. PMA, H2O2, and O2•- increased ROS production in human macrophages, generating oxidative stress in bacteria and augmented the gene expression of whiB3, lip-Y, Icl-1, and tgs-1. Our results suggest that ROS production in macrophages induces oxidative stress in intracellular bacteria inducing whiB3 expression. This factor may activate the synthesis of reserve lipids produced to survive in the latency state, which allows its persistence for long periods within the host.
Keywords: Mycobacterium tuberculosis; WhiB3; lipid metabolism; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response.PLoS Pathog. 2009 Aug;5(8):e1000545. doi: 10.1371/journal.ppat.1000545. Epub 2009 Aug 14. PLoS Pathog. 2009. PMID: 19680450 Free PMC article.
-
Mycobacterium tuberculosis WhiB3 Responds to Vacuolar pH-induced Changes in Mycothiol Redox Potential to Modulate Phagosomal Maturation and Virulence.J Biol Chem. 2016 Feb 5;291(6):2888-903. doi: 10.1074/jbc.M115.684597. Epub 2015 Dec 4. J Biol Chem. 2016. PMID: 26637353 Free PMC article.
-
Mycobacterium tuberculosis arrests host cycle at the G1/S transition to establish long term infection.PLoS Pathog. 2017 May 22;13(5):e1006389. doi: 10.1371/journal.ppat.1006389. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542477 Free PMC article.
-
Not too fat to fight: The emerging role of macrophage fatty acid metabolism in immunity to Mycobacterium tuberculosis.Immunol Rev. 2021 May;301(1):84-97. doi: 10.1111/imr.12952. Epub 2021 Feb 8. Immunol Rev. 2021. PMID: 33559209 Review.
-
Mycobacterium tuberculosis WhiB3: a novel iron-sulfur cluster protein that regulates redox homeostasis and virulence.Antioxid Redox Signal. 2012 Apr 1;16(7):687-97. doi: 10.1089/ars.2011.4341. Antioxid Redox Signal. 2012. PMID: 22010944 Free PMC article. Review.
Cited by
-
WhiB-like proteins: Diversity of structure, function and mechanism.Biochim Biophys Acta Mol Cell Res. 2024 Oct;1871(7):119787. doi: 10.1016/j.bbamcr.2024.119787. Epub 2024 Jun 13. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 38879133 Review.
-
Prominent transcriptomic changes in Mycobacterium intracellulare under acidic and oxidative stress.BMC Genomics. 2024 Apr 17;25(1):376. doi: 10.1186/s12864-024-10292-4. BMC Genomics. 2024. PMID: 38632539 Free PMC article.
-
Mycobacterium tuberculosis-macrophage interaction: Molecular updates.Front Cell Infect Microbiol. 2023 Mar 3;13:1062963. doi: 10.3389/fcimb.2023.1062963. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936766 Free PMC article. Review.
-
Uncovering the roles of Mycobacterium tuberculosis melH in redox and bioenergetic homeostasis: implications for antitubercular therapy.mSphere. 2024 Apr 23;9(4):e0006124. doi: 10.1128/msphere.00061-24. Epub 2024 Apr 2. mSphere. 2024. PMID: 38564709 Free PMC article.
-
Gene Regulatory Mechanism of Mycobacterium Tuberculosis during Dormancy.Curr Issues Mol Biol. 2024 Jun 11;46(6):5825-5844. doi: 10.3390/cimb46060348. Curr Issues Mol Biol. 2024. PMID: 38921019 Free PMC article. Review.
References
-
- World Health Organization . Global Tuberkulosis Report. World Health Organization; Geneva, Switzerland: 2021.
-
- Brugarolas P., Movahedzadeh F., Wang Y., Zhang N., Bartek I.L., Gao Y.N., Voskuil M.I., Franzblau S.G., He C. The Oxidation-Sensing Regulator (MosR) Is a New Redox-Dependent Transcription Factor in Mycobacterium Tuberculosis. J. Biol. Chem. 2012;287:37703–37712. doi: 10.1074/jbc.M112.388611. - DOI - PMC - PubMed
-
- Khan M.Z., Bhaskar A., Upadhyay S., Kumari P., Rajmani R.S., Jain P., Singh A., Kumar D., Bhavesh N.S., Nandicoori V.K. Protein Kinase G Confers Survival Advantage to Mycobacterium Tuberculosis during Latency-like Conditions. J. Biol. Chem. 2017;292:16093–16108. doi: 10.1074/jbc.M117.797563. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

