Bovine Immunity and Vitamin D3: An Emerging Association in Johne's Disease
- PMID: 36144467
- PMCID: PMC9500906
- DOI: 10.3390/microorganisms10091865
Bovine Immunity and Vitamin D3: An Emerging Association in Johne's Disease
Abstract
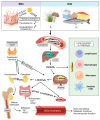
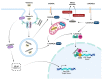
Mycobacterium avium subspecies paratuberculosis (MAP) is an environmentally hardy pathogen of ruminants that plagues the dairy industry. Hallmark clinical symptoms include granulomatous enteritis, watery diarrhea, and significant loss of body condition. Transition from subclinical to clinical infection is a dynamic process led by MAP which resides in host macrophages. Clinical stage disease is accompanied by dysfunctional immune responses and a reduction in circulating vitamin D3. The immunomodulatory role of vitamin D3 in infectious disease has been well established in humans, particularly in Mycobacterium tuberculosis infection. However, significant species differences exist between the immune system of humans and bovines, including effects induced by vitamin D3. This fact highlights the need for continued study of the relationship between vitamin D3 and bovine immunity, especially during different stages of paratuberculosis.
Keywords: Mycobacterium avium subsp. paratuberculosis; PBMC; cattle; endosomal trafficking; macrophage; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Exogenous Vitamin D3 Modulates Response of Bovine Macrophages to Mycobacterium avium subsp. paratuberculosis Infection and Is Dependent Upon Stage of Johne's Disease.Front Cell Infect Microbiol. 2022 Jan 17;11:773938. doi: 10.3389/fcimb.2021.773938. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35111692 Free PMC article.
-
Vitamin D3 alters macrophage phenotype and endosomal trafficking markers in dairy cattle naturally infected with Mycobacterium avium subsp. paratuberculosis.Front Cell Infect Microbiol. 2022 Oct 5;12:1021657. doi: 10.3389/fcimb.2022.1021657. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36275033 Free PMC article.
-
Stage of infection with Mycobacterium avium subsp. paratuberculosis impacts expression of Rab5, Rab7, and CYP27B1 in macrophages within the ileum of naturally infected cows.Front Vet Sci. 2023 Feb 3;10:1117591. doi: 10.3389/fvets.2023.1117591. eCollection 2023. Front Vet Sci. 2023. PMID: 36816182 Free PMC article.
-
Johne's disease in Canada Part I: clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds.Can Vet J. 2006 Sep;47(9):874-82. Can Vet J. 2006. PMID: 17017652 Free PMC article. Review.
-
Potential application of emerging diagnostic techniques to the diagnosis of bovine Johne's disease (paratuberculosis).Vet J. 2016 Mar;209:32-9. doi: 10.1016/j.tvjl.2015.10.033. Epub 2015 Oct 22. Vet J. 2016. PMID: 26831164 Review.
References
-
- Gao L.-Y., Laval F., Lawson E.H., Groger R.K., Woodruff A., Morisaki J.H., Cox J.S., Daffe M., Brown E.J. Requirement for kasB in Mycobacterium Mycolic Acid Biosynthesis, Cell Wall Impermeability and Intracellular Survival: Implications for Therapy. Mol. Microbiol. 2003;49:1547–1563. doi: 10.1046/j.1365-2958.2003.03667.x. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

