Antibacterial and Anti-Inflammatory Activities of Thymus vulgaris Essential Oil Nanoemulsion on Acne Vulgaris
- PMID: 36144477
- PMCID: PMC9503056
- DOI: 10.3390/microorganisms10091874
Antibacterial and Anti-Inflammatory Activities of Thymus vulgaris Essential Oil Nanoemulsion on Acne Vulgaris
Abstract
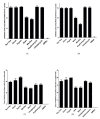
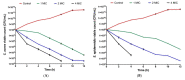
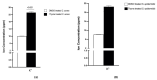
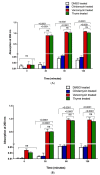
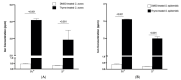
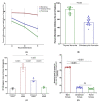
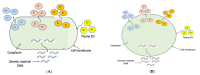
Antibiotics are frequently used in acne treatment and their prolonged use has led to an emergence of resistance. This study aimed to investigate the use of natural antimicrobials as an alternative therapy. The antimicrobial and anti-inflammatory activities of five commonly used essential oils (EOs) (tea tree, clove, thyme, mentha and basil EOs), and their possible mechanisms of action against Cutibacterium acnes and Staphylococcus epidermidis, were explored. The effect of the most potent EO on membrane permeability was elucidated and its anti-inflammatory action, when formulated as nanoemulsion, was tested in an in vivo acne model. The in vitro studies showed that thyme EO had the most potent antimicrobial and antibiofilm activity, with phenolics and terpenoids as main antimicrobial constituents of EO. Thyme EO affected cell membrane permeability of both bacterial species, evident by the detection of the leakage of intracellular ions and membrane integrity by the leakage of nucleic acids. Morphological alteration in bacterial cells was confirmed by transmission electron microscopy. Thyme EO nanoemulsion led to the suppression of an inflammatory response in acne animal models along with a bacterial load decrease and positive histopathological changes. Collectively, thyme EO nanoemulsion showed potent antimicrobial and anti-inflammatory effects compared to the reference antibiotics, suggesting its effectiveness as a natural alternative in acne treatment.
Keywords: Cutibacterium acnes; Staphylococcus epidermidis; acne vulgaris; antibiotic resistance; biofilms; inflammation; mode of action; nanoparticles; natural antimicrobials; plant extracts.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Nagy I., Pivarcsi A., Kis K., Koreck A., Bodai L., McDowell A., Kemény L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–2205. doi: 10.1016/j.micinf.2006.04.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

