Application of CO2 Supercritical Fluid to Optimize the Solubility of Oxaprozin: Development of Novel Machine Learning Predictive Models
- PMID: 36144490
- PMCID: PMC9506598
- DOI: 10.3390/molecules27185762
Application of CO2 Supercritical Fluid to Optimize the Solubility of Oxaprozin: Development of Novel Machine Learning Predictive Models
Abstract
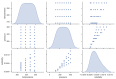
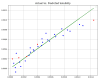
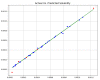
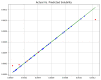
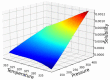
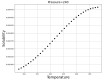
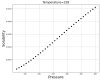
Over the last years, extensive motivation has emerged towards the application of supercritical carbon dioxide (SCCO2) for particle engineering. SCCO2 has great potential for application as a green and eco-friendly technique to reach small crystalline particles with narrow particle size distribution. In this paper, an artificial intelligence (AI) method has been used as an efficient and versatile tool to predict and consequently optimize the solubility of oxaprozin in SCCO2 systems. Three learning methods, including multi-layer perceptron (MLP), Kriging or Gaussian process regression (GPR), and k-nearest neighbors (KNN) are selected to make models on the tiny dataset. The dataset includes 32 data points with two input parameters (temperature and pressure) and one output (solubility). The optimized models were tested with standard metrics. MLP, GPR, and KNN have error rates of 2.079 × 10-8, 2.173 × 10-9, and 1.372 × 10-8, respectively, using MSE metrics. Additionally, in terms of R-squared, they have scores of 0.868, 0.997, and 0.999, respectively. The optimal inputs are the same as the maximum possible values and are paired with a solubility of 1.26 × 10-3 as an output.
Keywords: green chemistry; machine learning; mathematical modeling; optimization; solubility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Design of predictive model to optimize the solubility of Oxaprozin as nonsteroidal anti-inflammatory drug.Sci Rep. 2022 Jul 30;12(1):13106. doi: 10.1038/s41598-022-17350-5. Sci Rep. 2022. PMID: 35907929 Free PMC article.
-
Mathematical modeling and numerical simulation of supercritical processing of drug nanoparticles optimization for green processing: AI analysis.PLoS One. 2024 Sep 4;19(9):e0309242. doi: 10.1371/journal.pone.0309242. eCollection 2024. PLoS One. 2024. Retraction in: PLoS One. 2024 Dec 19;19(12):e0316403. doi: 10.1371/journal.pone.0316403. PMID: 39231157 Free PMC article. Retracted.
-
Solubility Optimization of Loxoprofen as a Nonsteroidal Anti-Inflammatory Drug: Statistical Modeling and Optimization.Molecules. 2022 Jul 7;27(14):4357. doi: 10.3390/molecules27144357. Molecules. 2022. PMID: 35889230 Free PMC article.
-
Supercritical carbon dioxide-based technologies for the production of drug nanoparticles/nanocrystals - A comprehensive review.Adv Drug Deliv Rev. 2018 Jun;131:22-78. doi: 10.1016/j.addr.2018.07.010. Epub 2018 Jul 17. Adv Drug Deliv Rev. 2018. PMID: 30026127 Review.
-
Recent advancements toward the incremsent of drug solubility using environmentally-friendly supercritical CO2: a machine learning perspective.Front Med (Lausanne). 2024 Sep 2;11:1467289. doi: 10.3389/fmed.2024.1467289. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39286644 Free PMC article. Review.
Cited by
-
Simulation and Optimization: A New Direction in Supercritical Technology Based Nanomedicine.Bioengineering (Basel). 2023 Dec 8;10(12):1404. doi: 10.3390/bioengineering10121404. Bioengineering (Basel). 2023. PMID: 38135995 Free PMC article. Review.
References
-
- Tabernero A., del Valle E.M.M., Galán M.A. Supercritical fluids for pharmaceutical particle engineering: Methods, basic fundamentals and modelling. Chem. Eng. Process. Process Intensif. 2012;60:9–25.
-
- Zhuang W., Hachem K., Bokov D., Ansari M.J., Nakhjiri A.T. Ionic liquids in pharmaceutical industry: A systematic review on applications and future perspectives. J. Mol. Liq. 2021;349:118145.
-
- Scherließ R., Bock S., Bungert N., Neustock A., Valentin L. Particle engineering in dry powders for inhalation. Eur. J. Pharm. Sci. 2022;172:106158. - PubMed
-
- Girotra P., Singh S.K., Nagpal K. Supercritical fluid technology: A promising approach in pharmaceutical research. Pharm. Dev. Technol. 2013;18:22–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

