Aziridination Reactivity of a Manganese(II) Complex with a Bulky Chelating Bis(Alkoxide) Ligand
- PMID: 36144492
- PMCID: PMC9505844
- DOI: 10.3390/molecules27185751
Aziridination Reactivity of a Manganese(II) Complex with a Bulky Chelating Bis(Alkoxide) Ligand
Abstract
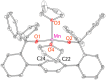
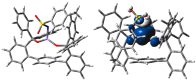
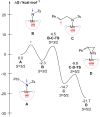
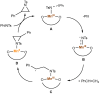
Treatment of Mn(N(SiMe3)2)2(THF)2 with bulky chelating bis(alkoxide) ligand [1,1':4',1''-terphenyl]-2,2''-diylbis(diphenylmethanol) (H2[O-terphenyl-O]Ph) formed a seesaw manganese(II) complex Mn[O-terphenyl-O]Ph(THF)2, characterized by structural, spectroscopic, magnetic, and analytical methods. The reactivity of Mn[O-terphenyl-O]Ph(THF)2 with various nitrene precursors was investigated. No reaction was observed between Mn[O-terphenyl-O]Ph(THF)2 and aryl azides. In contrast, the treatment of Mn[O-terphenyl-O]Ph(THF)2 with iminoiodinane PhINTs (Ts = p-toluenesulfonyl) was consistent with the formation of a metal-nitrene complex. In the presence of styrene, the reaction led to the formation of aziridine. Combining varying ratios of styrene and PhINTs in different solvents with 10 mol% of Mn[O-terphenyl-O]Ph(THF)2 at room temperature produced 2-phenylaziridine in up to a 79% yield. Exploration of the reactivity of Mn[O-terphenyl-O]Ph(THF)2 with various olefins revealed (1) moderate aziridination yields for p-substituted styrenes, irrespective of the electronic nature of the substituent; (2) moderate yield for 1,1'-disubstituted α-methylstyrene; (3) no aziridination for aliphatic α-olefins; (4) complex product mixtures for the β-substituted styrenes. DFT calculations suggest that iminoiodinane is oxidatively added upon binding to Mn, and the resulting formal imido intermediate has a high-spin Mn(III) center antiferromagnetically coupled to an imidyl radical. This imidyl radical reacts with styrene to form a sextet intermediate that readily reductively eliminates the formation of a sextet Mn(II) aziridine complex.
Keywords: alkoxides; aziridination; iminoiodinane; manganese.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis of Chromium(II) Complexes with Chelating Bis(alkoxide) Ligand and Their Reactions with Organoazides and Diazoalkanes.Molecules. 2020 Jan 9;25(2):273. doi: 10.3390/molecules25020273. Molecules. 2020. PMID: 31936557 Free PMC article.
-
Catalytic C-H bond amination from high-spin iron imido complexes.J Am Chem Soc. 2011 Apr 6;133(13):4917-23. doi: 10.1021/ja110066j. Epub 2011 Mar 15. J Am Chem Soc. 2011. PMID: 21405138
-
A Mechanistic Study on Iron-Based Styrene Aziridination: Understanding Epoxidation via Nitrene Hydrolysis.Molecules. 2024 Jul 24;29(15):3470. doi: 10.3390/molecules29153470. Molecules. 2024. PMID: 39124875 Free PMC article.
-
High-valent iron and manganese complexes of corrole and porphyrin in atom transfer and dioxygen evolving catalysis.Dalton Trans. 2011 Apr 14;40(14):3435-44. doi: 10.1039/c0dt01341b. Epub 2011 Jan 31. Dalton Trans. 2011. PMID: 21279237 Review.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
Cited by
-
Synthesis, Characterization, and the Effect of Lewis Bases on the Nuclearity of Iron Alkoxide Complexes.Inorg Chem. 2024 Apr 29;63(17):7692-7704. doi: 10.1021/acs.inorgchem.3c04538. Epub 2024 Apr 12. Inorg Chem. 2024. PMID: 38608180 Free PMC article.
-
Efficient carbene transfer reactivity mediated by Fe(II) complexes supported by bulky alkoxides.Chem Commun (Camb). 2024 Jul 4;60(55):7033-7036. doi: 10.1039/d4cc02108h. Chem Commun (Camb). 2024. PMID: 38896088 Free PMC article.
References
-
- Sweeney J.B. Aziridines: Epoxides’ Ugly Cousins? Chem. Soc. Rev. 2002;31:247–258. - PubMed
-
- Paike V.V., Kauthale S.S., Kumar R.C., Katariya M.V., More R.R., Ameta K.L., Mane S.B. Aziridines: Synthesis and bioactivity. In: Ameta K.L., Pawar R.P., Domb A.J., editors. Bioactive Heterocycles. Nova Scientific Publishers; Hauppauge, NY, USA: 2013. pp. 41–68.
-
- Kametani T., Honda T. Application of Aziridines to the Synthesis of Natural Products. Adv. Heterocycl. Chem. 1986;39:181–236.
-
- Werner L., Machara A., Sullivan B., Carrera I., Moser M., Adams D.R., Hudlicky T. Several Generations of Chemoenzymatic Synthesis of Oseltamivir (Tamiflu): Evolution of Strategy, Quest for a Process-Quality Synthesis, and Evaluation of Efficiency Metrics. J. Org. Chem. 2011;76:10050–10067. - PubMed
-
- Hanessian S., Guesne S., Chenard E. Total Synthesis of “Aliskiren”: The First Renin Inhibitor in Clinical Practice for Hypertension. Org. Lett. 2010;12:1816–1819. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

