Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation
- PMID: 36144512
- PMCID: PMC9504594
- DOI: 10.3390/molecules27185776
Interfacial Characterization of Polypyrrole/AuNP Composites towards Electrocatalysis of Ascorbic Acid Oxidation
Abstract
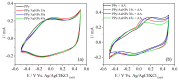
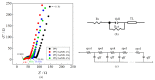
Polypyrrole (PPy) is an interesting conducting polymer due to its good environmental stability, high conductivity, and biocompatibility. The association between PPy and metallic nanoparticles has been widely studied since it enhances electrochemical properties. In this context, gold ions are reduced to gold nanoparticles (AuNPs) directly on the polymer surface as PPy can be oxidized to an overoxidized state. This work proposes the PPy electrochemical synthesis followed by the direct reduction of gold on its surface in a fast reaction. The modified electrodes were characterized by electronic microscopic and infrared spectroscopy. The effect of reduction time on the electrochemical properties was evaluated by the electrocatalytic properties of the obtained material from the oxidation of ascorbic acid (AA) and electrochemical impedance spectroscopy studies. The presence of AuNPs improved the AA electrocatalysis by reducing oxidation potential and lowering charge transfer resistance. EIS data were fitted using a transmission line model. The results indicated an increase in the electronic transport of the polymeric film in the presence of AuNPs. However, PPy overoxidation occurs when the AuNPs' deposition is higher than 30 s. In PPy/AuNPs 15 s, smaller and less agglomerated particles were formed with fewer PPy overoxidized, confirming the observed electrocatalytic behavior.
Keywords: conducting polymers; electrocatalysis; electrochemical impedance spectroscopy; gold nanoparticles; overoxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Bredas J.L., Street G.B. Polarons, Bipolarons, and Solitons in Conducting Polymers. Acc. Chem. Res. 1985;18:309–315. doi: 10.1021/ar00118a005. - DOI
-
- Wang J., Hui N. Electrochemical Functionalization of Polypyrrole Nanowires for the Development of Ultrasensitive Biosensors for Detecting MicroRNA. Sens. Actuators B Chem. 2019;281:478–485. doi: 10.1016/j.snb.2018.10.131. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

