Morin-VitaminE-β-CyclodextrinInclusionComplexLoadedChitosanNanoparticles (M-Vit.E-CD-CSNPs) Ameliorate Arsenic-Induced Hepatotoxicityina Murine Model
- PMID: 36144555
- PMCID: PMC9504860
- DOI: 10.3390/molecules27185819
Morin-VitaminE-β-CyclodextrinInclusionComplexLoadedChitosanNanoparticles (M-Vit.E-CD-CSNPs) Ameliorate Arsenic-Induced Hepatotoxicityina Murine Model
Abstract
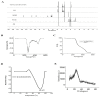
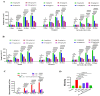
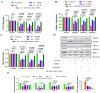
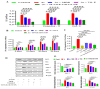
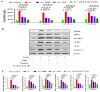
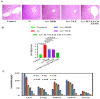
The special features of cyclodextrins (CDs), hydrophilic outer surfaces and hydrophobic inner surfaces, allow for development of inclusion complexes. The two bioactive strong antioxidant hepatoprotective compounds, Morin and vitamin E, are water insoluble. The present study aimed to prepare Morin-vitamin E-β-cyclodextrin inclusion complex loaded chitosan nanoparticles (M-Vit.E-CD-CS NPs) and to examine their hepatoprotective efficacy against arsenic-induced toxicity in a murine model. The NPs were characterized by FTIR, DLS, NMR, DSC, XRD, AFM, and a TEM study. The NPs were spherical in shape, 178 ± 1.5 nm in size with a polydispersity index (PDI) value of 0.18 and a zeta potential value of −22.4 ± 0.31 mV, with >50% encapsulation and drug loading efficacy. Mice were exposed to arsenic via drinking water, followed by treatment without or with the NPs on every alternate day up to 30 days by oral gavaging. Administration of NPs inhibited the arsenic-induced elevation of liver function markers, inflammatory and proapoptotic factors, reactive oxygen species (ROS) production, alteration in the level of blood parameters and antioxidant factors, and liver damage, which was measured by different biochemical assays, ELISA, Western blot, and histological study. Organ distribution of nanoparticles was measured by HPLC. M-Vit.E-CD-CS NPs showing potent hepatoprotective activity may be therapeutically beneficial.
Keywords: Morin; apoptosis; chitosan; hepatoprotection; inflammation; nanoformulation; oxidative stress; vitamin E; β-cyclodextrin.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Sunil A.A., Nadagouda N.M., Tejraj M.A. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release. 2004;100:5–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

