Enzymatic and Molecular Characterization of Anti- Leishmania Molecules That Differently Target Leishmania and Mammalian eIF4A Proteins, LieIF4A and eIF4AMus
- PMID: 36144626
- PMCID: PMC9502374
- DOI: 10.3390/molecules27185890
Enzymatic and Molecular Characterization of Anti- Leishmania Molecules That Differently Target Leishmania and Mammalian eIF4A Proteins, LieIF4A and eIF4AMus
Abstract
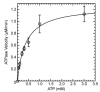
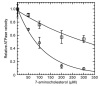
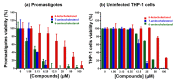
Previous investigations of the Leishmania infantum eIF4A-like protein (LieIF4A) as a potential drug target delivered cholestanol derivatives inhibitors. Here, we investigated the mode of action of cholesterol derivatives as a novel scaffold structure of LieIF4A inhibitors on the RNA-dependent ATPase activity of LieIF4A and its mammalian ortholog (eIF4AI). We compared their biochemical effects on RNA-dependent ATPase activities of both proteins and investigated if rocaglamide, a known inhibitor of eIF4A, could affect LieIF4A as well. Kinetic measurements were conducted at different concentrations of ATP, of the compound and in the presence of saturating whole yeast RNA concentrations. Kinetic analyses showed different ATP binding affinities for the two enzymes as well as different sensitivities to 7-α-aminocholesterol and rocaglamide. The 7-α-aminocholesterol inhibited LieIF4A with a higher binding affinity relative to cholestanol analogs. Cholesterol, another tested sterol, had no effect on the ATPase activity of LieIF4A or eIF4AI. The 7-α-aminocholesterol demonstrated an anti-Leishmania activity on L. infantum promastigotes. Additionally, docking simulations explained the importance of the double bond between C5 and C6 in 7-α-aminocholesterol and the amino group in the C7 position. In conclusion, Leishmania and mammalian eIF4A proteins appeared to interact differently with effectors, thus making LieIF4A a potential drug against leishmaniases.
Keywords: 7-α-aminocholesterol; Leishmania infantum; drug design; inhibitor; translation-initiation factor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
Figures
Similar articles
-
Distinct modes of interaction within eIF4F-like complexes and susceptibility to the RocA inhibitor for the Trypanosoma brucei EIF4AI translation initiation factor.PLoS One. 2025 May 9;20(5):e0322812. doi: 10.1371/journal.pone.0322812. eCollection 2025. PLoS One. 2025. PMID: 40343969 Free PMC article.
-
The steroid derivative 6-aminocholestanol inhibits the DEAD-box helicase eIF4A (LieIF4A) from the Trypanosomatid parasite Leishmania by perturbing the RNA and ATP binding sites.Mol Biochem Parasitol. 2018 Dec;226:9-19. doi: 10.1016/j.molbiopara.2018.10.001. Epub 2018 Oct 23. Mol Biochem Parasitol. 2018. PMID: 30365976
-
Leishmania infantum LeIF protein is an ATP-dependent RNA helicase and an eIF4A-like factor that inhibits translation in yeast.FEBS J. 2006 Nov;273(22):5086-100. doi: 10.1111/j.1742-4658.2006.05506.x. FEBS J. 2006. PMID: 17087726
-
The DEAD-box helicase eIF4A: paradigm or the odd one out?RNA Biol. 2013 Jan;10(1):19-32. doi: 10.4161/rna.21966. Epub 2012 Sep 20. RNA Biol. 2013. PMID: 22995829 Free PMC article. Review.
-
Small-Molecule Inhibitors Targeting eIF4A in Leukemia.Curr Protein Pept Sci. 2021;22(7):559-566. doi: 10.2174/1389203722666210526155808. Curr Protein Pept Sci. 2021. PMID: 34042032 Review.
Cited by
-
Distinct modes of interaction within eIF4F-like complexes and susceptibility to the RocA inhibitor for the Trypanosoma brucei EIF4AI translation initiation factor.PLoS One. 2025 May 9;20(5):e0322812. doi: 10.1371/journal.pone.0322812. eCollection 2025. PLoS One. 2025. PMID: 40343969 Free PMC article.
-
Approved drugs successfully repurposed against Leishmania based on machine learning predictions.Front Cell Infect Microbiol. 2024 Sep 26;14:1403589. doi: 10.3389/fcimb.2024.1403589. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39391884 Free PMC article.
-
Targeting translation initiation yields fast-killing therapeutics against the zoonotic parasite Cryptosporidium parvum.PLoS Pathog. 2025 Jul 28;21(7):e1012881. doi: 10.1371/journal.ppat.1012881. eCollection 2025 Jul. PLoS Pathog. 2025. PMID: 40720562 Free PMC article.
-
Phytosterols: Potential Therapeutic Effects and Challenges in Food Industry.Adv Exp Med Biol. 2024;1440:453-462. doi: 10.1007/978-3-031-43883-7_22. Adv Exp Med Biol. 2024. PMID: 38036893
References
-
- WHO 2021. [(accessed on 1 February 2022)]. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1.
-
- Chávez-Fumagalli M.A., Lage D.P., Tavares G.S.V., Mendonça D.V.C., Dias D.S., Ribeiro P.A.F., Ludolf F., Costa L.E., Coelho V.T.S., Coelho E.A.F. In silico Leishmania proteome mining applied to identify drug target potential to be used to treat against visceral and tegumentary leishmaniasis. ACS Infect. Dis. 2019;87:89–97. doi: 10.1016/j.jmgm.2018.11.014. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

