Effective Preparation of [18F]Flumazenil Using Copper-Mediated Late-Stage Radiofluorination of a Stannyl Precursor
- PMID: 36144667
- PMCID: PMC9505495
- DOI: 10.3390/molecules27185931
Effective Preparation of [18F]Flumazenil Using Copper-Mediated Late-Stage Radiofluorination of a Stannyl Precursor
Abstract
(1) Background: [18F]Flumazenil 1 ([18F]FMZ) is an established positron emission tomography (PET) radiotracer for the imaging of the gamma-aminobutyric acid (GABA) receptor subtype, GABAA in the brain. The production of [18F]FMZ 1 for its clinical use has proven to be challenging, requiring harsh radiochemical conditions, while affording low radiochemical yields. Fully characterized, new methods for the improved production of [18F]FMZ 1 are needed. (2) Methods: We investigate the use of late-stage copper-mediated radiofluorination of aryl stannanes to improve the production of [18F]FMZ 1 that is suitable for clinical use. Mass spectrometry was used to identify the chemical by-products that were produced under the reaction conditions. (3) Results: The radiosynthesis of [18F]FMZ 1 was fully automated using the iPhase FlexLab radiochemistry module, affording a 22.2 ± 2.7% (n = 5) decay-corrected yield after 80 min. [18F]FMZ 1 was obtained with a high radiochemical purity (>98%) and molar activity (247.9 ± 25.9 GBq/µmol). (4) Conclusions: The copper-mediated radiofluorination of the stannyl precursor is an effective strategy for the production of clinically suitable [18F]FMZ 1.
Keywords: Flumazenil 1; GABAA; PET imaging; benzodiazepine; radiochemistry; radiofluorination; stannane; stannyl.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
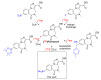


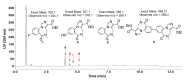
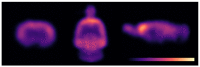
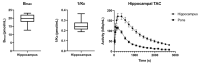
References
-
- Amrein R., Leishman B., Bentzinger C., Roncari G. Flumazenil in benzodiazepine antagonism. Actions and clinical use in intoxications and anaesthesiology. Med. Toxicol. Advers. Drug Exp. 1987;2:411–429. - PubMed
-
- Hoffman E.J., Warren E.W. Flumazenil: A benzodiazepine antagonist. Clin. Pharm. 1993;12:699–701. - PubMed
-
- Michel T.C. CHAPTER 12—Intravenous Anesthetics and Benzodiazepines. In: Duke J., editor. Anesthesia Secrets. 4th ed. Mosby; Maryland Heights, MI, USA: 2011. pp. 90–94.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

