Indane-1,3-Dione: From Synthetic Strategies to Applications
- PMID: 36144711
- PMCID: PMC9501146
- DOI: 10.3390/molecules27185976
Indane-1,3-Dione: From Synthetic Strategies to Applications
Abstract
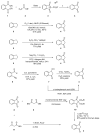
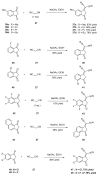
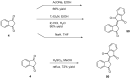
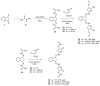
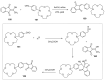
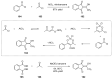
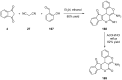
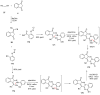
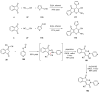
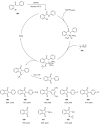
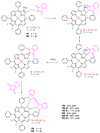
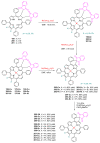
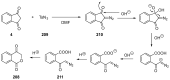
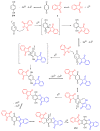
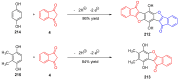
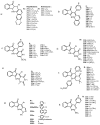
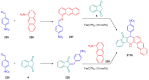
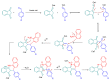
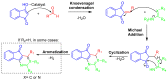
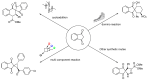
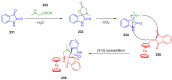
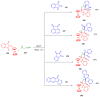
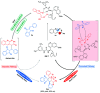
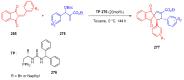
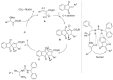
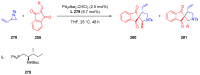
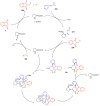
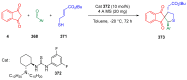
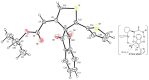
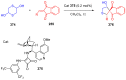
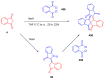
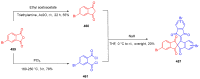
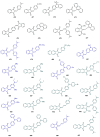
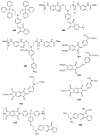
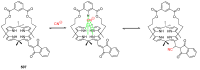
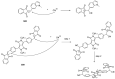
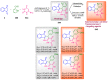
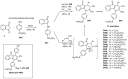
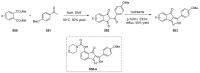
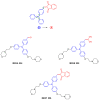
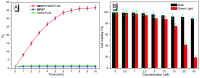
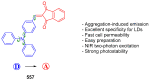
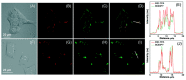
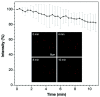
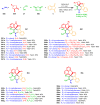
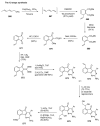
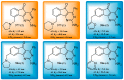
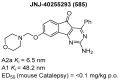
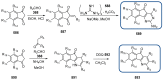
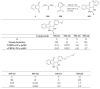
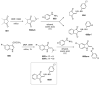
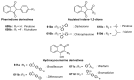
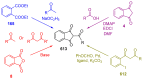
Indane-1,3-dione is a versatile building block used in numerous applications ranging from biosensing, bioactivity, bioimaging to electronics or photopolymerization. In this review, an overview of the different chemical reactions enabling access to this scaffold but also to the most common derivatives of indane-1,3-dione are presented. Parallel to this, the different applications in which indane-1,3-dione-based structures have been used are also presented, evidencing the versatility of this structure.
Keywords: MCR; chemical modification; domino reaction; indanedione; spiro compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


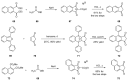
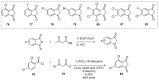
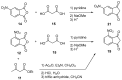
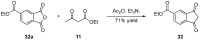
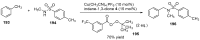
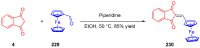



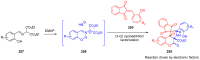
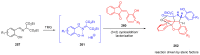
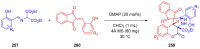
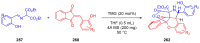
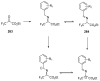
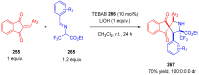

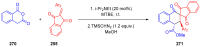
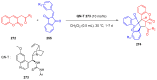



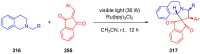
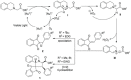
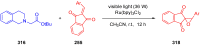


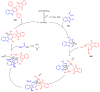
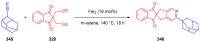
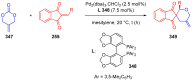
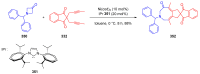

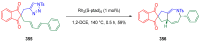
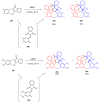
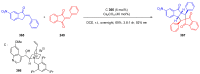
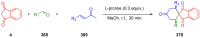


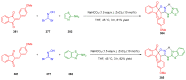
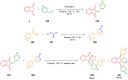
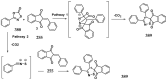
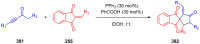





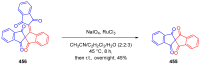


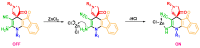
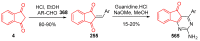

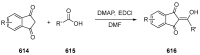
References
-
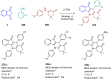
- Menezes J.C.J.M.D.S. Arylidene Indanone Scaffold: Medicinal Chemistry and Structure–Activity Relationship View. RSC Adv. 2017;7:9357–9372. doi: 10.1039/C6RA28613E. - DOI
-
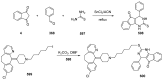
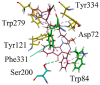
- Costanzo P., Cariati L., Desiderio D., Sgammato R., Lamberti A., Arcone R., Salerno R., Nardi M., Masullo M., Oliverio M. Design, Synthesis, and Evaluation of Donepezil-Like Compounds as AChE and BACE-1 Inhibitors. ACS Med. Chem. Lett. 2016;7:470–475. doi: 10.1021/acsmedchemlett.5b00483. - DOI - PMC - PubMed
-
- Vacca J.P., Dorsey B.D., Schleif W.A., Levin R.B., McDaniel S.L., Darke P.L., Zugay J., Quintero J.C., Blahy O.M., Roth E. L-735,524: An Orally Bioavailable Human Immunodeficiency Virus Type 1 Protease Inhibitor. Proc. Natl. Acad. Sci. USA. 1994;91:4096. doi: 10.1073/pnas.91.9.4096. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

