Spectroscopic Analysis of an Antimalarial Drug's (Quinine) Influence on Human Serum Albumin Reduction and Antioxidant Potential
- PMID: 36144764
- PMCID: PMC9505252
- DOI: 10.3390/molecules27186027
Spectroscopic Analysis of an Antimalarial Drug's (Quinine) Influence on Human Serum Albumin Reduction and Antioxidant Potential
Abstract
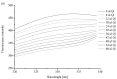
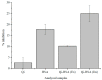
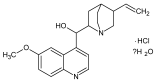
Quinine (Qi) is a well-known drug used in malaria therapy; it is also a potential anti-arrhythmic drug used in the treatment of calf cramps, rheumatoid arthritis, colds, and photodermatitis. Moreover, it is used in the food industry for the production of tonics. This study aimed to analyze the interaction between quinine and a transporting protein-human serum albumin (HSA)-as well as the influence of Qi on both protein reduction and antioxidant potential. It was found that Qi (via spectrofluorometric measurements and circular dichroism spectroscopy) binds to HSA with a low affinity and slightly affects the secondary structure of albumin. As demonstrated by the use of ABTS and FRAP assays, HSA has a higher antioxidant and reduction potential than Qi, while their mutual interaction results in a synergistic effect in antioxidant activity and reduction potential.
Keywords: antioxidant and reduction potential; human serum albumin; quinine; spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
New Synthetic Quinoline (Qui) Derivatives as Novel Antioxidants and Potential HSA's Antioxidant Activity Modulators-Spectroscopic Studies.Molecules. 2022 Dec 30;28(1):320. doi: 10.3390/molecules28010320. Molecules. 2022. PMID: 36615514 Free PMC article.
-
Recent Updates on Interaction Studies and Drug Delivery of Antimalarials with Serum Albumin Proteins.Curr Med Chem. 2024;31(25):3925-3953. doi: 10.2174/0929867330666230509121931. Curr Med Chem. 2024. PMID: 37218197 Review.
-
Molecular Insight into the Binding of Astilbin with Human Serum Albumin and Its Effect on Antioxidant Characteristics of Astilbin.Molecules. 2022 Jul 13;27(14):4487. doi: 10.3390/molecules27144487. Molecules. 2022. PMID: 35889360 Free PMC article.
-
Comparative investigation on interaction between potent antimalarials and human serum albumin using multispectroscopic and computational approaches.Luminescence. 2023 Dec;38(12):2018-2033. doi: 10.1002/bio.4590. Epub 2023 Sep 16. Luminescence. 2023. PMID: 37654050
-
Spectroscopic methodologies and molecular docking studies on the interaction of antimalarial drug piperaquine and its metabolites with human serum albumin.Spectrochim Acta A Mol Biomol Spectrosc. 2019 Nov 5;222:117158. doi: 10.1016/j.saa.2019.117158. Epub 2019 May 30. Spectrochim Acta A Mol Biomol Spectrosc. 2019. PMID: 31181505
Cited by
-
The effect of selected aminoglycoside antibiotics on human serum albumin antioxidant activity: a spectroscopic and calorimetric comparative study.Pharmacol Rep. 2023 Oct;75(5):1276-1290. doi: 10.1007/s43440-023-00529-6. Epub 2023 Sep 13. Pharmacol Rep. 2023. PMID: 37704832 Free PMC article.
-
New Synthetic Quinoline (Qui) Derivatives as Novel Antioxidants and Potential HSA's Antioxidant Activity Modulators-Spectroscopic Studies.Molecules. 2022 Dec 30;28(1):320. doi: 10.3390/molecules28010320. Molecules. 2022. PMID: 36615514 Free PMC article.
-
Recent Updates on Interaction Studies and Drug Delivery of Antimalarials with Serum Albumin Proteins.Curr Med Chem. 2024;31(25):3925-3953. doi: 10.2174/0929867330666230509121931. Curr Med Chem. 2024. PMID: 37218197 Review.
-
Ligand-human serum albumin analysis: the near-UV CD and UV-Vis spectroscopic studies.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar;398(3):3119-3131. doi: 10.1007/s00210-024-03471-3. Epub 2024 Sep 30. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39347800 Free PMC article.
-
A Novel Equipment-Free Paper-Based Fluorometric Method for the Analytical Determination of Quinine in Soft Drink Samples.Sensors (Basel). 2023 May 28;23(11):5153. doi: 10.3390/s23115153. Sensors (Basel). 2023. PMID: 37299880 Free PMC article.
References
-
- Permin H., Norn S., Kruse E., Kruse P.R. On the history of Cinchona bark in the treatment of Malaria. Dan. Medicinhist. Arbog. 2016;44:9–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

