Recent Advancements and Challenges in Lignin Valorization: Green Routes towards Sustainable Bioproducts
- PMID: 36144795
- PMCID: PMC9500909
- DOI: 10.3390/molecules27186055
Recent Advancements and Challenges in Lignin Valorization: Green Routes towards Sustainable Bioproducts
Abstract
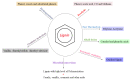
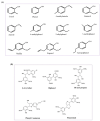
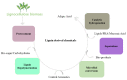
The aromatic hetero-polymer lignin is industrially processed in the paper/pulp and lignocellulose biorefinery, acting as a major energy source. It has been proven to be a natural resource for useful bioproducts; however, its depolymerization and conversion into high-value-added chemicals is the major challenge due to the complicated structure and heterogeneity. Conversely, the various pre-treatments techniques and valorization strategies offers a potential solution for developing a biomass-based biorefinery. Thus, the current review focus on the new isolation techniques for lignin, various pre-treatment approaches and biocatalytic methods for the synthesis of sustainable value-added products. Meanwhile, the challenges and prospective for the green synthesis of various biomolecules via utilizing the complicated hetero-polymer lignin are also discussed.
Keywords: biocatalysis; green synthesis; lignin; valorization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Al-Battashi H.S., Annamalai N., Sivakumar N., Al-Bahry S., Tripathi B.N., Nguyen Q.D., Gupta V.K. Lignocellulosic Biomass (LCB): A Potential Alternative Biorefinery Feedstock for Polyhydroxyalkanoates Production. Rev. Environ. Sci. Bio/Technol. 2019;18:183–205. doi: 10.1007/s11157-018-09488-4. - DOI
-
- Yousuf A., Pirozzi D., Sannino F. Fundamentals of Lignocellulosic Biomass. In: Yousuf A., Pirozzi D., Sannino F., editors. Lignocellulosic Biomass to Liquid Biofuels. Academic Press; Cambridge, MA, USA: 2020. pp. 1–15. Chapter 1.
-
- Huang J., Fu S., Gan L. Structure and Characteristics of Lignin. In: Huang J., Fu S., Gan L., editors. Lignin Chemistry and Applications. Elsevier; Amsterdam, The Netherlands: 2019. pp. 25–50. Chapter 2.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

