Improved Stability of Blue Colour of Anthocyanins from Lycium ruthenicum Murr. Based on Copigmentation
- PMID: 36144823
- PMCID: PMC9502443
- DOI: 10.3390/molecules27186089
Improved Stability of Blue Colour of Anthocyanins from Lycium ruthenicum Murr. Based on Copigmentation
Abstract
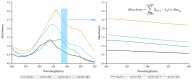
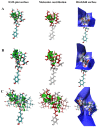
Natural blue food colourant is rare. The aim of this work was to screen compounds from the common copigments that could improve the blue tones of anthocyanins (ACNs) and to investigate the effect of different copigments on the colour stability of anthocyanins in neutral species. International Commission on Illumination (CIE) colour space, UV, IR, NMR, atomic force microscopy (AFM) and computational chemistry methods were utilised to evaluate ACNs from Lycium ruthenicum Murr. (LR), which is complexed with food additives and biological agents. The results indicate that Pro-Xylane (PX), Ectoin (ECT) and dipotassium glycyrrhizinate (DG) enhance the blue colour of the ACNs. ACNs-PX presents a colour close to Oxford Blue and has a surface height of 2.13 ± 0.14 nm and slightly improved stability. The half-life of ACNs-DG is improved 24.5-fold and had the highest complexation energy (-50.63/49.15) kcal/mol, indicating hydrogen bonds and π-π stacking forces enhance stability. These findings offer a new perspective for anthocyanin utilisation as a blue colourant and contribute to the large-scale application of LR.
Keywords: Lycium ruthenicum Murr.; anthocyanins; blue colour; copigmentation; stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Improved colorimetric analysis for subtle changes of powdered anthocyanins extracted from Lycium ruthenicum Murr.Food Chem. 2022 Mar 1;371:131080. doi: 10.1016/j.foodchem.2021.131080. Epub 2021 Sep 8. Food Chem. 2022. PMID: 34537620
-
In Situ Stability of Anthocyanins in Lycium ruthenicum Murray.Molecules. 2021 Nov 23;26(23):7073. doi: 10.3390/molecules26237073. Molecules. 2021. PMID: 34885653 Free PMC article.
-
Stabilization of Lycium ruthenicum Murr. anthocyanins by natural polyphenol extracts.J Food Sci. 2021 Oct;86(10):4365-4375. doi: 10.1111/1750-3841.15888. Epub 2021 Aug 24. J Food Sci. 2021. PMID: 34431095
-
Isolation, structure and biological activity of polysaccharides from the fruits of Lycium ruthenicum Murr: A review.Carbohydr Polym. 2022 Sep 1;291:119618. doi: 10.1016/j.carbpol.2022.119618. Epub 2022 May 13. Carbohydr Polym. 2022. PMID: 35698413 Review.
-
Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment.Chem Rev. 2016 May 11;116(9):4937-82. doi: 10.1021/acs.chemrev.5b00507. Epub 2016 Mar 9. Chem Rev. 2016. PMID: 26959943 Review.
Cited by
-
Influence of Copigmentation and Encapsulation on Stability and Antioxidant Activity of Anthocyanins from Blue and Pink Cornflower (Centaurea cyanus L.) Flowers.Molecules. 2025 Mar 26;30(7):1467. doi: 10.3390/molecules30071467. Molecules. 2025. PMID: 40286073 Free PMC article.
-
Characterization Variation of the Differential Coloring Substances in Rapeseed Petals with Different Colors Using UPLC-HESI-MS/MS.Molecules. 2023 Jul 26;28(15):5670. doi: 10.3390/molecules28155670. Molecules. 2023. PMID: 37570640 Free PMC article.
References
-
- Neves M.I.L., Silva E.K., Meireles M.A.A. Natural blue food colorants: Consumer acceptance, current alternatives, trends, challenges, and future strategies. Trends Food Sci. Technol. 2021;112:163–173. doi: 10.1016/j.tifs.2021.03.023. - DOI
MeSH terms
Substances
Grants and funding
- LGC20B020001 and LQ21C020002/Zhejiang Provincial Natural Science Foundation
- 2020-SF-A2/Qinghai Provincial Science Foundation
- Qinghai Province High-end Innovative Thousand Talents Program
- Youth Innovation Promotion Association CAS
- 2020-0407-NCC-0001/Innovation Platform for the Development and Construction of Special Project of Qinghai Province
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

