Production of Salvianic Acid A from l-DOPA via Biocatalytic Cascade Reactions
- PMID: 36144828
- PMCID: PMC9501478
- DOI: 10.3390/molecules27186088
Production of Salvianic Acid A from l-DOPA via Biocatalytic Cascade Reactions
Abstract
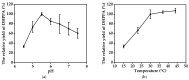
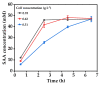
Salvianic acid A (SAA), as the main bioactive component of the traditional Chinese herb Salvia miltiorrhiza, has important application value in the treatment of cardiovascular diseases. In this study, a two-step bioprocess for the preparation of SAA from l-DOPA was developed. In the first step, l-DOPA was transformed to 3,4-dihydroxyphenylalanine (DHPPA) using engineered Escherichia coli cells expressing membrane-bound L-amino acid deaminase from Proteus vulgaris. After that, the unpurified DHPPA was directly converted into SAA by permeabilized recombinant E. coli cells co-expressing d-lactate dehydrogenase from Pediococcus acidilactici and formate dehydrogenase from Mycobacterium vaccae N10. Under optimized conditions, 48.3 mM of SAA could be prepared from 50 mM of l-DOPA, with a yield of 96.6%. Therefore, the bioprocess developed here was not only environmentally friendly, but also exhibited excellent production efficiency and, thus, is promising for industrial SAA production.
Keywords: biocatalysis; biological engineering; l-DOPA; membrane-bound l-amino acid deaminases; molecular biology; salvianic acid A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Development of a Multienzyme Cascade for Salvianolic Acid A Synthesis from l-Tyrosine.ACS Omega. 2025 Jan 31;10(5):4792-4800. doi: 10.1021/acsomega.4c09986. eCollection 2025 Feb 11. ACS Omega. 2025. PMID: 39959076 Free PMC article.
-
Chromosome engineering of Escherichia coli for constitutive production of salvianic acid A.Microb Cell Fact. 2017 May 16;16(1):84. doi: 10.1186/s12934-017-0700-2. Microb Cell Fact. 2017. PMID: 28511681 Free PMC article.
-
Metabolic engineering of Escherichia coli for production of salvianic acid A via an artificial biosynthetic pathway.Metab Eng. 2013 Sep;19:79-87. doi: 10.1016/j.ymben.2013.06.001. Epub 2013 Jun 14. Metab Eng. 2013. PMID: 23774671
-
Production of phenylpyruvic acid by engineered L-amino acid deaminase from Proteus mirabilis.Biotechnol Lett. 2022 Jun;44(5-6):635-642. doi: 10.1007/s10529-022-03245-y. Epub 2022 Apr 16. Biotechnol Lett. 2022. PMID: 35429303 Review.
-
Incorporating unnatural amino acids to engineer biocatalysts for industrial bioprocess applications.Biotechnol J. 2015 Dec;10(12):1862-76. doi: 10.1002/biot.201500153. Epub 2015 Sep 24. Biotechnol J. 2015. PMID: 26399851 Review.
Cited by
-
Efficient production of salvianic acid A from L-dihydroxyphenylalanine through a tri-enzyme cascade.Bioresour Bioprocess. 2023 May 1;10(1):31. doi: 10.1186/s40643-023-00649-0. Bioresour Bioprocess. 2023. PMID: 38647923 Free PMC article.
-
Development of a Multienzyme Cascade for Salvianolic Acid A Synthesis from l-Tyrosine.ACS Omega. 2025 Jan 31;10(5):4792-4800. doi: 10.1021/acsomega.4c09986. eCollection 2025 Feb 11. ACS Omega. 2025. PMID: 39959076 Free PMC article.
References
-
- Zhao B.L., Jiang W., Zhao Y., Hou J.W., Xin W.J. Scavenging Effects of Salvia Miltiorrhiza on Free Radicals and Its Protection for Myocardial Mitochondrial Membranes from Ischemia-Reperfusion Injury. Biochem. Mol. Biol. Int. 1996;38:1171–1182. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

