Synthesis and Biological Importance of 2-(thio)ureabenzothiazoles
- PMID: 36144837
- PMCID: PMC9502297
- DOI: 10.3390/molecules27186104
Synthesis and Biological Importance of 2-(thio)ureabenzothiazoles
Abstract
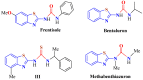
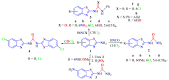
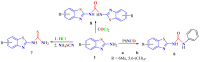
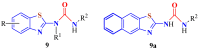
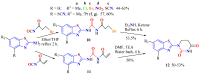
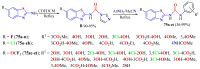
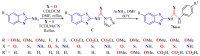
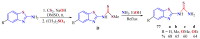
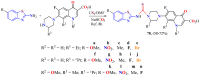
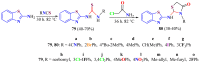
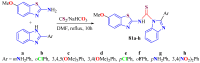
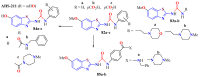
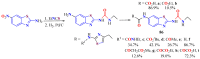
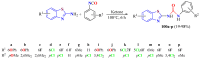
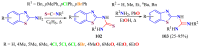
The (thio)urea and benzothiazole (BT) derivatives have been shown to have a broad spectrum of biological activities. These groups, when bonded, result in the 2-(thio)ureabenzothizoles (TBT and UBT), which could favor the physicochemical and biological properties. UBTs and TBTs are compounds of great importance in medicinal chemistry. For instance, Frentizole is a UBT derivative used for the treatment of rheumatoid arthritis and systemic lupus erythematosus. The UBTs Bentaluron and Bethabenthiazuron are commercial fungicides used as wood preservatives and herbicides in winter corn crops. On these bases, we prepared this bibliography review, which covers chemical aspects of UBTs and TBTs as potential therapeutic agents as well as their studies on the mechanisms of a variety of pharmacological activities. This work covers synthetic methodologies from 1935 to nowadays, highlighting the most recent approaches to afford UBTs and TBTs with a variety of substituents as illustrated in 42 schemes and 13 figures and concluded with 187 references. In addition, this interesting review is designed on chemical reactions of 2-aminobenzothiazoles (2ABTs) with (thio)phosgenes, iso(thio)cyanates, 1,1'-(thio)carbonyldiimidazoles [(T)CDI]s, (thio)carbamoyl chlorides, and carbon disulfide. This topic will provide information of utility for medicinal chemists dedicated to the design and synthesis of this class of compounds to be tested with respect to their biological activities and be proposed as new pharmacophores.
Keywords: (thio)carbonyldiimidazoles; (thio)phosgene; (thio)ureabenzothiazoles; 2-aminobenzothiazoles; carbamoyl chlorides; carbon disulfide; iso(thio)cyanates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
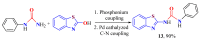

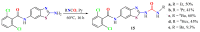
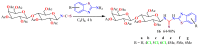









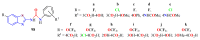
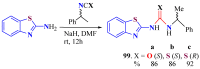

Similar articles
-
Design, Synthesis and Biological Activities of (Thio)Urea Benzothiazole Derivatives.Int J Mol Sci. 2023 May 30;24(11):9488. doi: 10.3390/ijms24119488. Int J Mol Sci. 2023. PMID: 37298442 Free PMC article. Review.
-
New synthesis and biological evaluation of benzothiazole derivates as antifungal agents.J Agric Food Chem. 2015 Apr 15;63(14):3681-6. doi: 10.1021/acs.jafc.5b00150. Epub 2015 Apr 1. J Agric Food Chem. 2015. PMID: 25797910
-
Rearrangements of acyl, thioacyl, and imidoyl (thio)cyanates to iso(thio)cyanates, acyl iso(thio)cyanates to (thio)acyl isocyanates, and imidoyl iso(thio)cyanates to (thio)acyl carbodiimides, RCX-YCN ⇌ RCX-NCY ⇌ RCY-NCX ⇌ RCY-XCN (X and Y = O, S, NR').J Org Chem. 2013 Mar 1;78(5):1802-10. doi: 10.1021/jo3013786. Epub 2012 Sep 20. J Org Chem. 2013. PMID: 22954414
-
Synthetic Procedures to Access 2-Guanidinobenzazoles of Biological Interest.Curr Org Synth. 2023;20(5):504-522. doi: 10.2174/1570179419666220615143529. Curr Org Synth. 2023. PMID: 35708075 Review.
-
A Comprehensive Review on Recent advances in Synthesis & Pharmacotherapeutic potential of Benzothiazoles.Antiinflamm Antiallergy Agents Med Chem. 2015;14(2):98-112. doi: 10.2174/1871523014666150528110703. Antiinflamm Antiallergy Agents Med Chem. 2015. PMID: 26017385 Review.
Cited by
-
In Silico and In Vivo Evaluation of Novel 2-Aminobenzothiazole Derivative Compounds as Antidiabetic Agents.Int J Mol Sci. 2025 Jan 22;26(3):909. doi: 10.3390/ijms26030909. Int J Mol Sci. 2025. PMID: 39940678 Free PMC article.
-
Design, Synthesis and Biological Activities of (Thio)Urea Benzothiazole Derivatives.Int J Mol Sci. 2023 May 30;24(11):9488. doi: 10.3390/ijms24119488. Int J Mol Sci. 2023. PMID: 37298442 Free PMC article. Review.
References
-
- Mokeev M.V., Sostanin S.A., Zuev V.V. Hydrogen Bonding in dicyclohexylmethane-or diphenylmethane based urea compounds and their polimer counterparts investigated by NMR spectroscopy: Interplay of electronic and geometrical factors. Chem. Phys. Lett. 2020;739:137047. doi: 10.1016/j.cplett.2019.137047. - DOI
-
- Lee J., La S., Ahn B.R., Jeong T.C., Kim D.H. Metabolism of 1-{3-[3-(4-cyanobenzyl)-3H-imidazol-4-yl]-propyl}-3-(6-methoxypyridin-3-yl)-1-(2-trifluoromethylbenzyl)-thiourea (YH3945), a novel anticancer drug, in rats using the14C-labeled compound. Rapid Commun. Mass Spectrom. 2004;18:1901–1910. doi: 10.1002/rcm.1555. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

