Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna minor
- PMID: 36144862
- PMCID: PMC9502776
- DOI: 10.3390/molecules27186123
Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna minor
Abstract
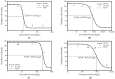
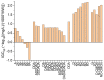
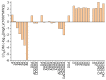
Chitosan, chitooligosaccharides and their derivatives’ production and use in many fields may result in their release to the environment, possibly affecting aquatic organisms. Both an experimental and a computational approach were considered for evaluating the effects of these compounds on Lemna minor. Based on the determined EC50 values against L. minor, only D-glucosamine hydrochloride (EC50 = 11.55 mg/L) was considered as “slightly toxic” for aquatic environments, while all the other investigated compounds, having EC50 > 100 mg/L, were considered as “practically non-toxic”. The results obtained in the experimental approach were in good agreement with the predictions obtained using the admetSAR2.0 computational tool, revealing that the investigated compounds were not considered toxic for crustacean, fish and Tetrahymena pyriformis aquatic microorganisms. The ADMETLab2.0 computational tool predicted the values of IGC50 for Tetrahymena pyriformis and the LC50 for fathead minnow and Daphnia magna, with the lowest values of these parameters being revealed by totally acetylated chitooligosaccharides in correlation with their lowest solubility. The effects of the chitooligosaccharides and chitosan on L. minor decreased with increased molecular weight, increased with the degree of deacetylation and were reliant on acetylation patterns. Furthermore, the solubility mainly influenced the effects on the aqueous environment, with a higher solubility conducted to lower toxicity.
Keywords: Lemna minor; aqueous environment; chitooligosaccharides; chitosan; ecotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zhao L.-M., Shi L.-E., Zhang Z.-L., Chen J.-M., Shi D.-D., Yang J., Tang Z.-X. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011;28:353–362. doi: 10.1590/S0104-66322011000300001. - DOI
-
- Morin-Crini N., Lichtfouse E., Torri G., Crini G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019;17:1667–1692. doi: 10.1007/s10311-019-00904-x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

