Photocatalytic Water Splitting Promoted by 2D and 3D Porphyrin Covalent Organic Polymers Synthesized by Suzuki-Miyaura Carbon-Carbon Coupling
- PMID: 36144987
- PMCID: PMC9503735
- DOI: 10.3390/nano12183197
Photocatalytic Water Splitting Promoted by 2D and 3D Porphyrin Covalent Organic Polymers Synthesized by Suzuki-Miyaura Carbon-Carbon Coupling
Abstract
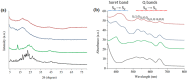
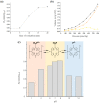
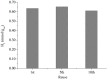
This work deals with the synthesis of metal-free and porphyrin-based covalent organic polymers (COPs) by the Suzuki-Miyaura coupling carbon-carbon bond forming reaction to study the photocatalytic overall water splitting performance. Apart from using 5,10,15,20-Tetrakis-(4-bromophenyl)porphyrin, we have chosen different cross-linker monomers to induce 2-dimensional (2D) or 3-dimensional (3D) and different rigidity in their resulting polymeric molecular structure. The synthesised COPs were extensively characterised to reveal that the dimensionality and flexibility of the molecular structure play an intense role in the physical, photochemical, and electronic properties of the polymers. Photoinduced excited state of the COPs was evaluated by nanosecond time-resolved laser transient absorption spectroscopy (TAS) by analysing excited state kinetics and quenching experiments, photocurrent density measurements and photocatalytic deposition of Ru3+ to RuO2, and photocatalysis. In summary, TAS experiments demonstrated that the transient excited state of these polymers has two decay kinetics and exhibit strong interaction with water molecules. Moreover, photocurrent and photocatalytic deposition experiments proved that charges are photoinduced and are found across the COP molecular network, but more important charges can migrate from the surface of the COP to the medium. Among the various COPs tested, COP-3 that has a flexible and 3D molecular structure reached the best photocatalytic performances, achieving a photocatalytic yield of 0.4 mmol H2 × gCOP-3-1 after 3 h irradiation.
Keywords: 2D; 3D; 5,10,15,20-Tetrakis-(4-bromophenyl)porphyrin; Suzuki–Miyaura coupling reaction; covalent organic polymers; hydrogen generation; oxygen generation; photoactive polymers; photocatalysis; photocatalytic water splitting; porphyrin; solar fuels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
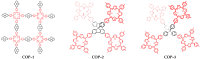




Similar articles
-
Porphyrin-based covalent organic polymers with customizable photoresponses for photodynamic inactivation of bacteria.Sci Total Environ. 2024 Jan 1;906:167475. doi: 10.1016/j.scitotenv.2023.167475. Epub 2023 Oct 4. Sci Total Environ. 2024. PMID: 37797764
-
Vinylene-Linked Fully Conjugated 3D Covalent Organic Polymers for Photocatalytic H2O2 Production.Chemistry. 2025 Jul 11;31(39):e202501480. doi: 10.1002/chem.202501480. Epub 2025 Jun 17. Chemistry. 2025. PMID: 40501030
-
Mixed-Blocks-Driving Nickel-Porphyrin Covalent Organic Polymers for Photocatalytic Syngas Production from CO2 with Fine Selectivity.ACS Appl Mater Interfaces. 2023 Aug 30;15(34):40519-40528. doi: 10.1021/acsami.3c07609. Epub 2023 Aug 21. ACS Appl Mater Interfaces. 2023. PMID: 37607045
-
2D Polymers as Emerging Materials for Photocatalytic Overall Water Splitting.Adv Mater. 2018 Nov;30(48):e1801955. doi: 10.1002/adma.201801955. Epub 2018 Jul 22. Adv Mater. 2018. PMID: 30033628 Review.
-
Amorphous and Crystalline 2D Polymeric Carbon Nitride Nanosheets for Photocatalytic Hydrogen/Oxygen Evolution and Hydrogen Peroxide Production.Chem Asian J. 2020 Aug 3;15(15):2329-2340. doi: 10.1002/asia.202000253. Epub 2020 Apr 27. Chem Asian J. 2020. PMID: 32291899 Review.
Cited by
-
Blue light-activated 5,10,15,20-tetrakis(4-bromophenyl)porphyrin for photodynamic eradication of drug-resistant Staphylococcus aureus.RSC Adv. 2024 Dec 18;14(53):39779-39786. doi: 10.1039/d4ra07666d. eCollection 2024 Dec 10. RSC Adv. 2024. PMID: 39697839 Free PMC article.
-
TM-Free and TM-Catalyzed Mechanosynthesis of Functional Polymers.Polymers (Basel). 2023 Apr 12;15(8):1853. doi: 10.3390/polym15081853. Polymers (Basel). 2023. PMID: 37112002 Free PMC article. Review.
-
Influence of the Polymeric Matrix on the Optical and Electrical Properties of Copper Porphine-Based Semiconductor Hybrid Films.Polymers (Basel). 2023 Jul 22;15(14):3125. doi: 10.3390/polym15143125. Polymers (Basel). 2023. PMID: 37514514 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

