Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies
- PMID: 36145149
- PMCID: PMC9504511
- DOI: 10.3390/nu14183773
Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies
Abstract
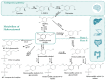
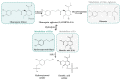
Consumption of olive products has been established as a health-promoting dietary pattern due to their high content in compounds with eminent pharmacological properties and well-described bioactivities. However, their metabolism has not yet been fully described. The present critical review aimed to gather all scientific data of the past two decades regarding the absorption and metabolism of the foremost olive compounds, specifically of the phenylalcohols hydroxytyrosol (HTyr) and tyrosol (Tyr) and the secoiridoids oleacein (Olea), oleocanthal (Oleo) and oleuropein (Oleu). A meticulous record of the in vitro assays and in vivo (animals and humans) studies of the characteristic olive compounds was cited, and a critical discussion on their bioavailability and metabolism was performed taking into account data from their gut microbial metabolism. The existing critical review summarizes the existing knowledge regarding the bioavailability and metabolism of olive-characteristic phenylalchohols and secoiridoids and spotlights the lack of data for specific chemical groups and compounds. Critical observations and conclusions were derived from correlating structure with bioavailability data, while results from in vitro, animal and human studies were compared and discussed, giving significant insight to the future design of research approaches for the total bioavailability and metabolism exploration thereof.
Keywords: ADMET properties; human studies; hydroxytyrosol; in vitro assays; in vivo; metabolism; oleacein; oleocanthal; oleuropein; tyrosol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Angelis A., Hamzaoui M., Aligiannis N., Nikou T., Michailidis D., Gerolimatos P., Termentzi A., Hubert J., Halabalaki M., Renault J.-H., et al. An integrated process for the recovery of high added-value compounds from olive oil using solid support free liquid-liquid extraction and chromatography techniques. J. Chromatogr. A. 2017;1491:126–136. doi: 10.1016/j.chroma.2017.02.046. - DOI - PubMed
-
- Rodríguez-López P., Lozano-Sanchez J., Borrás-Linares I., Emanuelli T., Menéndez J.A., Segura-Carretero A. Structure–biological activity relationships of extra-virgin olive oil phenolic compounds: Health properties and bioavailability. Antioxidants. 2020;9:685. doi: 10.3390/antiox9080685. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

