Synthesis, Characterisation and Mechanism of Action of Anticancer 3-Fluoroazetidin-2-ones
- PMID: 36145265
- PMCID: PMC9501633
- DOI: 10.3390/ph15091044
Synthesis, Characterisation and Mechanism of Action of Anticancer 3-Fluoroazetidin-2-ones
Abstract
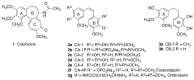
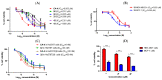
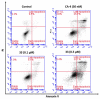
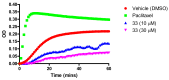
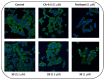
The stilbene combretastatin A-4 (CA-4) is a potent microtubule-disrupting agent interacting at the colchicine-binding site of tubulin. In the present work, the synthesis, characterisation and mechanism of action of a series of 3-fluoro and 3,3-difluoro substituted β-lactams as analogues of the tubulin-targeting agent CA-4 are described. The synthesis was achieved by a convenient microwave-assisted Reformatsky reaction and is the first report of 3-fluoro and 3,3-difluoro β-lactams as CA-4 analogues. The β-lactam compounds 3-fluoro-4-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxy phenyl)azetidin-2-one 32 and 3-fluoro-4-(3-fluoro-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one) 33 exhibited potent activity in MCF-7 human breast cancer cells with IC50 values of 0.075 µM and 0.095 µM, respectively, and demonstrated low toxicity in non-cancerous cells. Compound 32 also demonstrated significant antiproliferative activity at nanomolar concentrations in the triple-negative breast cancer cell line Hs578T (IC50 0.033 μM), together with potency in the invasive isogenic subclone Hs578Ts(i)8 (IC50 = 0.065 μM), while 33 was also effective in MDA-MB-231 cells (IC50 0.620 μM). Mechanistic studies demonstrated that 33 inhibited tubulin polymerisation, induced apoptosis in MCF-7 cells, and induced a downregulation in the expression of anti-apoptotic Bcl2 and survivin with corresponding upregulation in the expression of pro-apoptotic Bax. In silico studies indicated the interaction of the compounds with the colchicine-binding site, demonstrating the potential for further developing novel cancer therapeutics as microtubule-targeting agents.
Keywords: Reformatsky reaction; antiproliferative activity; breast cancer; combretastatin A-4; fluorinated β-lactams; inhibition of tubulin polymerisation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
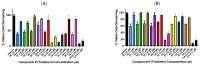

Similar articles
-
Antiproliferative and Tubulin-Destabilising Effects of 3-(Prop-1-en-2-yl)azetidin-2-Ones and Related Compounds in MCF-7 and MDA-MB-231 Breast Cancer Cells.Pharmaceuticals (Basel). 2023 Jul 13;16(7):1000. doi: 10.3390/ph16071000. Pharmaceuticals (Basel). 2023. PMID: 37513912 Free PMC article.
-
Synthesis and Antiproliferative Evaluation of 3-Chloroazetidin-2-ones with Antimitotic Activity: Heterocyclic Bridged Analogues of Combretastatin A-4.Pharmaceuticals (Basel). 2021 Oct 31;14(11):1119. doi: 10.3390/ph14111119. Pharmaceuticals (Basel). 2021. PMID: 34832901 Free PMC article.
-
Synthesis by diastereomeric resolution, biochemical evaluation and molecular modelling of chiral 3-hydroxyl b-lactam microtubule-targeting agents for the treatment of triple negative breast and chemoresistant colorectal cancers.Bioorg Chem. 2023 Dec;141:106877. doi: 10.1016/j.bioorg.2023.106877. Epub 2023 Sep 30. Bioorg Chem. 2023. PMID: 37804699
-
β-Lactams with antiproliferative and antiapoptotic activity in breast and chemoresistant colon cancer cells.Eur J Med Chem. 2020 Mar 1;189:112050. doi: 10.1016/j.ejmech.2020.112050. Epub 2020 Jan 10. Eur J Med Chem. 2020. PMID: 31954879
-
3-Vinylazetidin-2-Ones: Synthesis, Antiproliferative and Tubulin Destabilizing Activity in MCF-7 and MDA-MB-231 Breast Cancer Cells.Pharmaceuticals (Basel). 2019 Apr 11;12(2):56. doi: 10.3390/ph12020056. Pharmaceuticals (Basel). 2019. PMID: 30979033 Free PMC article.
Cited by
-
Antiproliferative and Tubulin-Destabilising Effects of 3-(Prop-1-en-2-yl)azetidin-2-Ones and Related Compounds in MCF-7 and MDA-MB-231 Breast Cancer Cells.Pharmaceuticals (Basel). 2023 Jul 13;16(7):1000. doi: 10.3390/ph16071000. Pharmaceuticals (Basel). 2023. PMID: 37513912 Free PMC article.
-
DataPype: A Fully Automated Unified Software Platform for Computer-Aided Drug Design.ACS Omega. 2023 Oct 12;8(42):39468-39480. doi: 10.1021/acsomega.3c05207. eCollection 2023 Oct 24. ACS Omega. 2023. PMID: 37901539 Free PMC article.
-
Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy.Biomolecules. 2022 Dec 10;12(12):1843. doi: 10.3390/biom12121843. Biomolecules. 2022. PMID: 36551271 Free PMC article. Review.
References
-
- World Health Organization Breast Cancer. [(accessed on 3 May 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
-
- Li X., Yang J., Peng L., Sahin A.A., Huo L., Ward K.C., O’Regan R., Torres M.A., Meisel J.L. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res. Treat. 2017;161:279–287. doi: 10.1007/s10549-016-4059-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

