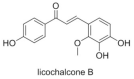
Licochalcone B, a Natural Autophagic Agent for Alleviating Oxidative Stress-Induced Cell Death in Neuronal Cells and Caenorhabditis elegans Models
- PMID: 36145273
- PMCID: PMC9502728
- DOI: 10.3390/ph15091052
Licochalcone B, a Natural Autophagic Agent for Alleviating Oxidative Stress-Induced Cell Death in Neuronal Cells and Caenorhabditis elegans Models
Abstract
Autophagy has been implicated in the regulation of neuroinflammation and neurodegenerative disorders. Licochalcone B (LCB), a chalcone from Glycyrrhiza inflata, has been reported to have anti-cancer, anti-oxidation and anti-β-amyloid fibrillation effects; however, its effect in autophagy remain un-investigated. In the current study, the potential neuro-protective role of LCB in terms of its anti-oxidative, anti-apoptotic, and autophagic properties upon oxidative stress-induced damage in neuronal cells was investigated. With the production of reactive oxygen species (ROS) as a hallmark of neuroinflammation and neurodegeneration, hydrogen peroxide (H2O2) was adopted to stimulate ROS-induced cell apoptosis in PC-12 cells. Our findings revealed that LCB reduced cell cytotoxicity and apoptosis of PC-12 cells upon H2O2-stimulation. Furthermore, LCB increased the level of the apoptosis-associated proteins caspase-3 and cleaved caspase-3 in H2O2-induced cells. LCB effectively attenuated the level of oxidative stress markers such as MDA, SOD, and ROS in H2O2-induced cells. Most importantly, LCB was confirmed to possess its anti-apoptotic effects in H2O2-induced cells through the induction of ATG7-dependent autophagy and the SIRT1/AMPK signaling pathway. As a novel autophagic inducer, LCB increased the level of autophagy-related proteins LC3-II and decreased p62 in both neuronal cells and Caenorhabditis elegans (C. elegans) models. These results suggested that LCB has potential neuroprotective effects on oxidative damage models via multiple protective pharmacological mechanisms.
Keywords: Licochalcone B; antioxidant; apoptosis; autophagy; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

