Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies
- PMID: 36145281
- PMCID: PMC9506496
- DOI: 10.3390/ph15091060
Achillea fragrantissima (Forssk.) Sch.Bip Flower Dichloromethane Extract Exerts Anti-Proliferative and Pro-Apoptotic Properties in Human Triple-Negative Breast Cancer (MDA-MB-231) Cells: In Vitro and In Silico Studies
Abstract
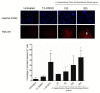
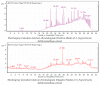
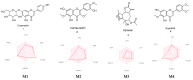
The aggressive triple-negative breast cancer (TNBC) is a challenging disease due to the absence of tailored therapy. The search for new therapies involves intensive research focusing on natural sources. Achillea fragrantissima (A. fragrantissima) is a traditional medicine from the Middle East region. Various solvent extracts from different A. fragrantissima plant parts, including flowers, leaves, and roots, were tested on TNBC MDA-MB-231 cells. Using liquid chromatography, the fingerprinting revealed rich and diverse compositions for A. fragrantissima plant parts using polar to non-polar solvent extracts indicating possible differences in bioactivities. Using the CellTiter-Glo™ viability assay, the half-maximal inhibitory concentration (IC50) values were determined for each extract and ranged from 32.4 to 161.7 µg/mL. The A. fragrantissima flower dichloromethane extract had the lowest mean IC50 value and was chosen for further investigation. Upon treatment with increasing A. fragrantissima flower dichloromethane extract concentrations, the MDA-MB-231 cells displayed, in a dose-dependent manner, enhanced morphological and biochemical hallmarks of apoptosis, including cell shrinkage, phosphatidylserine exposure, caspase activity, and mitochondrial outer membrane permeabilization, assessed using phase-contrast microscopy, fluorescence-activated single-cell sorting analysis, Image-iT™ live caspase, and mitochondrial transition pore opening activity, respectively. Anticancer target prediction and molecular docking studies revealed the inhibitory activity of a few A. fragrantissima flower dichloromethane extract-derived metabolites against carbonic anhydrase IX, an enzyme reported for its anti-apoptotic properties. In conclusion, these findings suggest promising therapeutic values of the A. fragrantissima flower dichloromethane extract against TNBC development.
Keywords: Achillea fragrantissima; carbonic anhydrase; caspase activation; mitochondrial apoptosis pathway; triple-negative breast cancer.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures













Similar articles
-
Achillea fragrantissima (Forssk.) Sch.Bip instigates the ROS/FADD/c-PARP expression that triggers apoptosis in breast cancer cell (MCF-7).PLoS One. 2024 May 31;19(5):e0304072. doi: 10.1371/journal.pone.0304072. eCollection 2024. PLoS One. 2024. PMID: 38820323 Free PMC article.
-
Achillea fragrantissima (Forssk.) Sch.Bip. methanolic extract exerts potent antimicrobial activity and causes cancer cell death via induction of caspase-dependent apoptosis and S-phase arrest.Nat Prod Res. 2022 Sep;36(18):4645-4650. doi: 10.1080/14786419.2021.2010074. Epub 2021 Nov 30. Nat Prod Res. 2022. PMID: 34847782
-
Comparative study of the essential oils and extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) from Egypt.Pharmazie. 2004 Mar;59(3):226-30. Pharmazie. 2004. PMID: 15074599
-
Anticancer potential of Phoenix dactylifera L. seed extract in human cancer cells and pro-apoptotic effects mediated through caspase-3 dependent pathway in human breast cancer MDA-MB-231 cells: an in vitro and in silico investigation.BMC Complement Med Ther. 2022 Mar 15;22(1):68. doi: 10.1186/s12906-022-03533-0. BMC Complement Med Ther. 2022. PMID: 35291987 Free PMC article.
-
Chloroform Fraction of Methanolic Extract of Seeds of Annona muricata Induce S Phase Arrest and ROS Dependent Caspase Activated Mitochondria-Mediated Apoptosis in Triple-Negative Breast Cancer.Anticancer Agents Med Chem. 2021;21(10):1250-1265. doi: 10.2174/1871520620666200918101448. Anticancer Agents Med Chem. 2021. PMID: 32951586
Cited by
-
Achillea fragrantissima (Forssk.) Sch.Bip instigates the ROS/FADD/c-PARP expression that triggers apoptosis in breast cancer cell (MCF-7).PLoS One. 2024 May 31;19(5):e0304072. doi: 10.1371/journal.pone.0304072. eCollection 2024. PLoS One. 2024. PMID: 38820323 Free PMC article.
-
Effect of Achillea fragrantissima Extract on Excision Wound Biofilms of MRSA and Pseudomonas aeruginosa in Diabetic Mice.Int J Mol Sci. 2023 Jun 5;24(11):9774. doi: 10.3390/ijms24119774. Int J Mol Sci. 2023. PMID: 37298725 Free PMC article.
-
Therapeutic potential of Achillea millefolium L. extract on 7,12-dimethylbenz(a)anthracene (DMBA) -induced ovary cancer in Wistar rats: a biochemical, molecular and histopathological approach.Toxicol Res (Camb). 2025 Jan 8;14(1):tfae235. doi: 10.1093/toxres/tfae235. eCollection 2025 Jan. Toxicol Res (Camb). 2025. PMID: 39790360
-
Silver nanoparticles synthesized using aerial part of Achillea fragrantissima and evaluation of their bioactivities.Sci Rep. 2024 Oct 21;14(1):24703. doi: 10.1038/s41598-024-75558-z. Sci Rep. 2024. PMID: 39433875 Free PMC article.
-
Advances in research on the intestinal microbiota in the mechanism and prevention of colorectal cancer (Review).Mol Med Rep. 2025 May;31(5):133. doi: 10.3892/mmr.2025.13498. Epub 2025 Mar 21. Mol Med Rep. 2025. PMID: 40116116 Free PMC article. Review.
References
-
- Li A., Keck J.M., Parmar S., Patterson J., Labrie M., Creason A.L., Johnson B.E., Downey M., Thomas G., Beadling C., et al. Characterizing advanced breast cancer heterogeneity and treatment resistance through serial biopsies and comprehensive analytics. NPJ Precis. Oncol. 2021;5:28. doi: 10.1038/s41698-021-00165-4. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

