Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids
- PMID: 36145292
- PMCID: PMC9500727
- DOI: 10.3390/ph15091071
Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids
Abstract
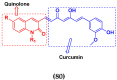
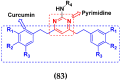
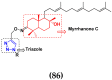
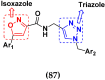
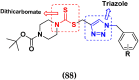
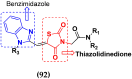
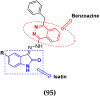
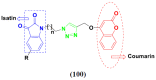
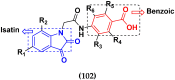
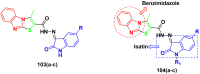
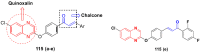
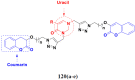
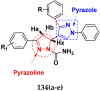
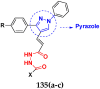
Cancer is a complex disease, and its treatment is a big challenge, with variable efficacy of conventional anticancer drugs. A two-drug cocktail hybrid approach is a potential strategy in recent drug discovery that involves the combination of two drug pharmacophores into a single molecule. The hybrid molecule acts through distinct modes of action on several targets at a given time with more efficacy and less susceptibility to resistance. Thus, there is a huge scope for using hybrid compounds to tackle the present difficulties in cancer medicine. Recent work has applied this technique to uncover some interesting molecules with substantial anticancer properties. In this study, we report data on numerous promising hybrid anti-proliferative/anti-tumor agents developed over the previous 10 years (2011-2021). It includes quinazoline, indole, carbazole, pyrimidine, quinoline, quinone, imidazole, selenium, platinum, hydroxamic acid, ferrocene, curcumin, triazole, benzimidazole, isatin, pyrrolo benzodiazepine (PBD), chalcone, coumarin, nitrogen mustard, pyrazole, and pyridine-based anticancer hybrids produced via molecular hybridization techniques. Overall, this review offers a clear indication of the potential benefits of merging pharmacophoric subunits from multiple different known chemical prototypes to produce more potent and precise hybrid compounds. This provides valuable knowledge for researchers working on complex diseases such as cancer.
Keywords: anticancer agents; cell lines; in vitro; molecular hybridization; pharmacophore.
Conflict of interest statement
The authors declare no competing interest.
Figures




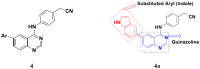
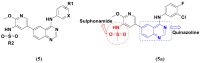
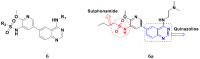

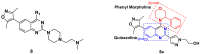


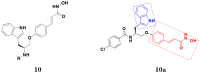


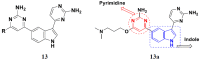









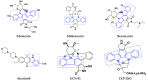

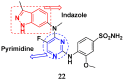







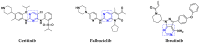
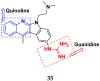
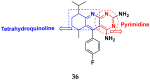



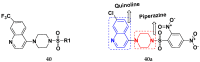
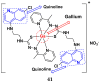
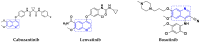


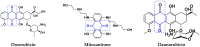
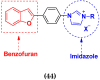










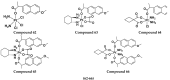
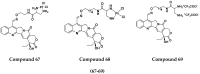

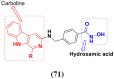







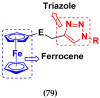





































References
-
- Rana A., Alex J.M., Chauhan M., Joshi G., Kumar R. A review on pharmacophoric designs of antiproliferative agents. Med. Chem. Res. 2015;24:903–920. doi: 10.1007/s00044-014-1196-5. - DOI
-
- Kori S. An overview: Several causes of breast cancer. Epidemol. Int. J. 2018;2:000107. doi: 10.23880/EIJ-16000107. - DOI
-
- Hassanpour S.H., Dehghani M. Review of cancer from perspective of molecular. J. Cancer Res. Pract. 2017;4:127–129. doi: 10.1016/j.jcrpr.2017.07.001. - DOI
-
- Philip C.C., Mathew A., John M.J. Cancer care: Challenges in the developing world. Cancer Res. Treat. 2018;1:58–62.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

