Potential Active Constituents from Opophytum forsskalii (Hochst. ex Boiss.) N.E.Br against Experimental Gastric Lesions in Rats
- PMID: 36145310
- PMCID: PMC9502456
- DOI: 10.3390/ph15091089
Potential Active Constituents from Opophytum forsskalii (Hochst. ex Boiss.) N.E.Br against Experimental Gastric Lesions in Rats
Abstract
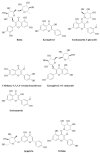
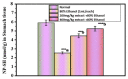
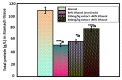
Opophytum forsskalii (O. forsskalii) is a desert plant that belongs to the Aizoaceae family. Although it is a natural food source for Bedouin tribes in northern Saudi Arabia, there is little information on its active metabolites. Therefore, the secondary metabolites of the hydroalcoholic extract from the leaves of this species were analyzed by liquid chromatography-mass chromatography (LC-MS). LC-MS identified a total of 30 secondary metabolites. These compounds represented two main categories among sixteen classes. Among them, flavonoids represented the largest proportion with eleven metabolites while fatty acids provided seven compounds. In addition, the extract was evaluated for its gastroprotective effect against gastric lesions induced by different models, such as indomethacin, stress, and necrotizing agents (80% ethanol, 0.2 mol/L NaOH, and 25% NaCl), in rats. For each method, group 1 was used as the control group while groups 2 and 3 received the leaf extract at doses of 200 and 400 mg/kg, respectively. The ulcer index (UI) and intraluminal bleeding score (IBS) were measured for each method. In addition, gastric tissue from the ethanol method was used for the analysis of nonprotein sulfhydrates (NP-SH), malondialdehyde (MDA), total protein (TP), and histopathologic evaluation. Pretreatment with O. forsskalii significantly decreased UI (p < 0.01) and IBS (p < 0.01) at 400 mg/kg. Pretreatment with O. forsskalii significantly improved total protein levels (p < 0.01) and NP-SH (p < 0.001) compared to the ethanol ulcer groups. MDA levels increased from 0.5 to 5.8 nmol/g in the normal groups compared to the ethanol groups and decreased to 2.34 nmol/g in the O. forsskalii pretreatment. In addition to the gastroprotective markers, histopathological examination of gastric tissue confirmed the gastroprotective potential of O. forsskalii extract against ethanol.
Keywords: Opophytum forsskalii; Saudi Arabia; gastro-protective; indomethacin; necrotizing agents; stress.
Conflict of interest statement
The author declares no conflict of interest.
Figures




Similar articles
-
Gastroprotective effect methanol extract of Caesalpinia coriaria pods against indomethacin- and ethanol-induced gastric lesions in Wistar rats.J Ethnopharmacol. 2023 Apr 6;305:116057. doi: 10.1016/j.jep.2022.116057. Epub 2022 Dec 24. J Ethnopharmacol. 2023. PMID: 36574790
-
Insights on the Mesembryanthemum forsskalii phenotype and study of the effects of several exogenous plant growth regulators via plant tissue culture.BMC Plant Biol. 2025 Jan 4;25(1):15. doi: 10.1186/s12870-024-06029-w. BMC Plant Biol. 2025. PMID: 39754075 Free PMC article.
-
Gastric antisecretory and antiulcer activity of bovine hemoglobin.World J Gastroenterol. 2013 Jun 7;19(21):3291-9. doi: 10.3748/wjg.v19.i21.3291. World J Gastroenterol. 2013. PMID: 23745031 Free PMC article.
-
Gastric Ulcer Healing Property of Bryophyllum pinnatum Leaf Extract in Chronic Model In Vivo and Gastroprotective Activity of Its Major Flavonoid.Front Pharmacol. 2021 Dec 16;12:744192. doi: 10.3389/fphar.2021.744192. eCollection 2021. Front Pharmacol. 2021. PMID: 34975468 Free PMC article.
-
Rocket "Eruca sativa": a salad herb with potential gastric anti-ulcer activity.World J Gastroenterol. 2009 Apr 28;15(16):1958-65. doi: 10.3748/wjg.15.1958. World J Gastroenterol. 2009. PMID: 19399927 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

