Microbiota Transplantation in Day-Old Broiler Chickens Ameliorates Necrotic Enteritis via Modulation of the Intestinal Microbiota and Host Immune Responses
- PMID: 36145404
- PMCID: PMC9503007
- DOI: 10.3390/pathogens11090972
Microbiota Transplantation in Day-Old Broiler Chickens Ameliorates Necrotic Enteritis via Modulation of the Intestinal Microbiota and Host Immune Responses
Abstract
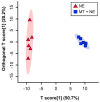
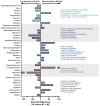
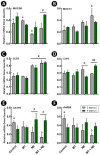
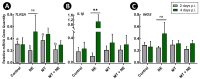
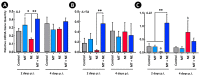
A microbiota transplant (MT) originating from mature adult chicken ceca and propagated in bioreactors was administered to day-old broiler chicks to ascertain the degree to which, and how, the MT affects Clostridium perfringens (Cp)-incited necrotic enteritis (NE). Using a stress predisposition model of NE, birds administered the MT and challenged with Cp showed fewer necrotic lesions, and exhibited a substantially higher α- and β-diversity of bacteria in their jejunum and ceca. Birds challenged with Cp and not administered the MT showed decreased Lactobacillus and increased Clostridium sensu strico 1 in the jejunum. In ceca, Megamonas, a genus containing butyrate-producing bacteria, was only present in birds administered the MT, and densities of this genus were increased in birds challenged with Cp. Metabolite profiles in cecal digesta were altered in birds administered the MT and challenged with the pathogen; 59 metabolites were differentially abundant following MT treatment, and the relative levels of short chain fatty acids, butyrate, valerate, and propionate, were decreased in birds with NE. Birds administered the MT and challenged with Cp showed evidence of enhanced restoration of intestinal barrier functions, including elevated mRNA of MUC2B, MUC13, and TJP1. Likewise, birds administered the MT exhibited higher mRNA of IL2, IL17A, and IL22 at 2-days post-inoculation with Cp, indicating that these birds were better immunologically equipped to respond to pathogen challenge. Collectively, study findings demonstrated that administering a MT containing a diverse mixture of microorganisms to day-old birds ameliorated NE in broilers by increasing bacterial diversity and promoting positive immune responses.
Keywords: Clostridium perfringens; immune responses; metabolomics; microbiota transplantation; necrotic enteritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens.Poult Sci. 2019 Dec 1;98(12):6422-6432. doi: 10.3382/ps/pez480. Poult Sci. 2019. PMID: 31424518 Free PMC article.
-
Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens.Poult Sci. 2019 Jul 1;98(7):2800-2812. doi: 10.3382/ps/pez084. Poult Sci. 2019. PMID: 30877749
-
Responses of broiler chickens orally challenged with Clostridium perfringens isolated from field cases of necrotic enteritis.Res Vet Sci. 2006 Aug;81(1):99-108. doi: 10.1016/j.rvsc.2005.10.006. Epub 2005 Dec 9. Res Vet Sci. 2006. PMID: 16337982
-
Research Note: Effect of synbiotic supplementation on caecal Clostridium perfringens load in broiler chickens with different necrotic enteritis challenge models.Poult Sci. 2020 May;99(5):2452-2458. doi: 10.1016/j.psj.2019.10.081. Epub 2020 Mar 18. Poult Sci. 2020. PMID: 32359580 Free PMC article.
-
Clostridium perfringens as Foodborne Pathogen in Broiler Production: Pathophysiology and Potential Strategies for Controlling Necrotic Enteritis.Animals (Basel). 2020 Sep 22;10(9):1718. doi: 10.3390/ani10091718. Animals (Basel). 2020. PMID: 32972009 Free PMC article. Review.
Cited by
-
Antimicrobial Growth Promoters Altered the Function but Not the Structure of Enteric Bacterial Communities in Broiler Chicks ± Microbiota Transplantation.Animals (Basel). 2023 Mar 9;13(6):997. doi: 10.3390/ani13060997. Animals (Basel). 2023. PMID: 36978538 Free PMC article.
-
Cecal Microbiota Development and Physiological Responses of Broilers Following Early Life Microbial Inoculation Using Different Delivery Methods and Microbial Sources.Appl Environ Microbiol. 2023 May 31;89(5):e0027123. doi: 10.1128/aem.00271-23. Epub 2023 Apr 26. Appl Environ Microbiol. 2023. PMID: 37098952 Free PMC article.
-
Recent advances in the application of microbiota transplantation in chickens.J Anim Sci Biotechnol. 2025 Jun 27;16(1):91. doi: 10.1186/s40104-025-01233-6. J Anim Sci Biotechnol. 2025. PMID: 40571924 Free PMC article. Review.
-
A comprehensive review of experimental models and induction protocols for avian necrotic enteritis over the past 2 decades.Front Vet Sci. 2024 Jul 24;11:1429637. doi: 10.3389/fvets.2024.1429637. eCollection 2024. Front Vet Sci. 2024. PMID: 39113718 Free PMC article. Review.
-
Infection by Salmonella enterica Serovar Typhimurium DT104 Modulates Immune Responses, the Metabolome, and the Function of the Enteric Microbiota in Neonatal Broiler Chickens.Pathogens. 2022 Oct 29;11(11):1257. doi: 10.3390/pathogens11111257. Pathogens. 2022. PMID: 36365008 Free PMC article.
References
-
- Dahiya J.P., Wilkie D.C., Van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 2006;129:60–88. doi: 10.1016/j.anifeedsci.2005.12.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

