Tuberculosis Treatment Response Monitoring by the Phenotypic Characterization of MTB-Specific CD4+ T-Cells in Relation to HIV Infection Status
- PMID: 36145465
- PMCID: PMC9506022
- DOI: 10.3390/pathogens11091034
Tuberculosis Treatment Response Monitoring by the Phenotypic Characterization of MTB-Specific CD4+ T-Cells in Relation to HIV Infection Status
Abstract
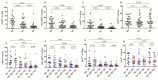
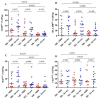
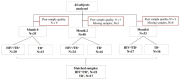
HIV infection causes systemic immune activation, impacts TB disease progression and hence may influence the diagnostic usability of Mycobacterium tuberculosis-specific T cell profiling. We investigated changes of activation and maturation markers on MTB-specific CD4+ T-cells after anti-tuberculosis treatment initiation in relation to HIV status and the severity of lung impairment. Thawed peripheral blood mononuclear cells from TB patients with (n = 27) and without HIV (n = 17) were analyzed using an intracellular IFN-γ assay and flow cytometry 2 and 6 months post-TB treatment initiation. H37Rv antigen was superior to the profile MTB-specific CD4+ T-cells phenotype when compared to PPD and ESAT6/CFP10. Regardless of HIV status and the severity of lung impairment, activation markers (CD38, HLA-DR and Ki67) on MTB-specific CD4+ T-cells declined after TB treatment initiation (p < 0.01), but the expression of the maturation marker CD27 did not change over the course of TB treatment. The MTB-specific T cell phenotype before, during and after treatment completion was similar between people living with and without HIV, as well as between subjects with severe and mild lung impairment. These data suggest that the assessment of activation and maturation markers on MTB-specific CD4+ T-cells can be useful for TB treatment monitoring, regardless of HIV status and the severity of lung disease.
Keywords: HIV; Mycobacterium tuberculosis (MTB)-specific; lung severity; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Phenotypic Changes on Mycobacterium Tuberculosis-Specific CD4 T Cells as Surrogate Markers for Tuberculosis Treatment Efficacy.Front Immunol. 2018 Sep 28;9:2247. doi: 10.3389/fimmu.2018.02247. eCollection 2018. Front Immunol. 2018. PMID: 30323818 Free PMC article.
-
Disease extent and anti-tubercular treatment response correlates with Mycobacterium tuberculosis-specific CD4 T-cell phenotype regardless of HIV-1 status.Clin Transl Immunology. 2020 Sep 28;9(9):e1176. doi: 10.1002/cti2.1176. eCollection 2020. Clin Transl Immunology. 2020. PMID: 33005414 Free PMC article.
-
Study of CD27, CD38, HLA-DR and Ki-67 immune profiles for the characterization of active tuberculosis, latent infection and end of treatment.Front Microbiol. 2022 Jul 22;13:885312. doi: 10.3389/fmicb.2022.885312. eCollection 2022. Front Microbiol. 2022. PMID: 35935194 Free PMC article.
-
Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis.Front Immunol. 2014 Apr 22;5:180. doi: 10.3389/fimmu.2014.00180. eCollection 2014. Front Immunol. 2014. PMID: 24795723 Free PMC article. Review.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
References
-
- World Health Organization . Global Tuberculosis Report 2021. World Health Organization; Geneva, Switzerland: 2021.
-
- Geldmacher C., Schuetz A., Ngwenyama N., Casazza J.P., Sanga E., Saathoff E., Boehme C., Geis S., Maboko L., Singh M., et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis. 2008;198:1590–1598. doi: 10.1086/593017. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

