Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques
- PMID: 36145466
- PMCID: PMC9503824
- DOI: 10.3390/pathogens11091033
Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques
Abstract
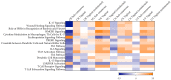
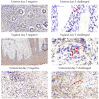
Zika virus (ZIKV) cases continue to be reported, and no vaccine or specific antiviral agent has been approved for the prevention or treatment of infection. Though ZIKV is primarily transmitted by mosquitos, cases of sexual transmission and prolonged viral RNA presence in semen have been reported. In this observational study, we report the mucosal responses to sub-cutaneous and mucosal ZIKV exposure in cynomolgus macaques during acute and late chronic infection. Subcutaneous challenge induced a decrease in the growth factor VEGF in colorectal and cervicovaginal tissues 100 days post-challenge, in contrast to the observed increase in these tissues following vaginal infection. This different pattern was not observed in the uterus, where VEGF was upregulated independently of the challenge route. Vaginal challenge induced a pro-inflammatory profile in all mucosal tissues during late chronic infection. Similar responses were already observed during acute infection in a vaginal tissue explant model of ex vivo challenge. Non-productive and productive infection 100 days post-in vivo vaginal challenge induced distinct proteomic profiles which were characterized by further VEGF increase and IL-10 decrease in non-infected animals. Ex vivo challenge of mucosal explants revealed tissue-specific modulation of cytokine levels during the acute phase of infection. Mucosal cytokine profiles could represent biosignatures of persistent ZIKV infection.
Keywords: Zika virus; ex vivo challenge; immune responses; mucosal tissue; non-human primates.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Maternal Zika Virus (ZIKV) Infection following Vaginal Inoculation with ZIKV-Infected Semen in Timed-Pregnant Olive Baboons.J Virol. 2020 May 18;94(11):e00058-20. doi: 10.1128/JVI.00058-20. Print 2020 May 18. J Virol. 2020. PMID: 32188737 Free PMC article.
-
Passive immunisation of convalescent human anti-Zika plasma protects against challenge with New World Zika virus in cynomolgus macaques.NPJ Vaccines. 2020 Sep 15;5:86. doi: 10.1038/s41541-020-00234-y. eCollection 2020. NPJ Vaccines. 2020. PMID: 33014434 Free PMC article.
-
Cellular and Humoral Immunity Protect against Vaginal Zika Virus Infection in Mice.J Virol. 2018 Mar 14;92(7):e00038-18. doi: 10.1128/JVI.00038-18. Print 2018 Apr 1. J Virol. 2018. PMID: 29343577 Free PMC article.
-
Zika Virus Infection, Reproductive Organ Targeting, and Semen Transmission in the Male Olive Baboon.J Virol. 2019 Dec 12;94(1):e01434-19. doi: 10.1128/JVI.01434-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597777 Free PMC article.
-
Zika virus as a sexually transmitted pathogen.Curr Opin Infect Dis. 2018 Feb;31(1):39-44. doi: 10.1097/QCO.0000000000000414. Curr Opin Infect Dis. 2018. PMID: 29176348 Review.
Cited by
-
A Framework for Understanding Maternal Immunity.Immunol Allergy Clin North Am. 2023 Feb;43(1S):e1-e20. doi: 10.1016/j.iac.2023.03.002. Epub 2023 Apr 15. Immunol Allergy Clin North Am. 2023. PMID: 37179052 Free PMC article. Review.
-
Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

