Respiratory Commensal Bacteria Increase Protection against Hypermucoviscous Carbapenem-Resistant Klebsiella pneumoniae ST25 Infection
- PMID: 36145495
- PMCID: PMC9501321
- DOI: 10.3390/pathogens11091063
Respiratory Commensal Bacteria Increase Protection against Hypermucoviscous Carbapenem-Resistant Klebsiella pneumoniae ST25 Infection
Abstract
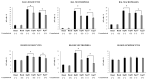
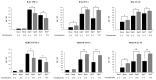
In a previous work, we demonstrated that nasally administered Corynebacterium pseudodiphtheriticum 090104 beneficially modulated the respiratory innate immune response and improved the protection against Respiratory Syncytial Virus and Streptococcus pneumoniae in mice. In this work, we aimed to evaluate whether the immunomodulatory 090104 strain was able to enhance the resistance against the respiratory infection induced by hypermucoviscous carbapenemase-producing (KPC-2) Klebsiella pneumoniae strains belonging to the sequence type (ST) 25. The nasal treatment of mice with C. pseudodiphtheriticum 090104 before the challenge with multiresistant K. pneumoniae ST25 strains significantly reduced lung bacterial cell counts and lung tissue damage. The protective effect of the 090104 strain was related to its ability to regulate the respiratory innate immune response triggered by K. pneumoniae challenge. C. pseudifteriticum 090104 differentially modulated the recruitment of leukocytes into the lung and the production of TNF-α, IFN-γ and IL-10 levels in the respiratory tract and serum. Our results make an advance in the positioning of C. pseudodiphtheriticum 090104 as a next-generation probiotic for the respiratory tract and encourage further research of this bacterium as a promising alternative to develop non-antibiotic therapeutical approaches to enhance the prevention of infections produced by microorganisms with multiple resistance to antimicrobials such as KPC-2-producing hypermucoviscous K. pneumoniae strains belonging to ST25.
Keywords: Corynebacterium pseudodiphtheriticum; Klebsiella pneumoniae; antibiotic resistance; immunobiotic; next generation probiotics; respiratory commensal bacteria; respiratory immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gomez S.A., Pasteran F.G., Faccone D., Tijet N., Rapoport M., Lucero C., Lastovetska O., Albornoz E., Galas M., KPC Group et al. Clonal dissemination of Klebsiella pneumoniae ST258 harbouring KPC-2 in Argentina. Clin. Microbiol. Infect. 2011;17:1520–1524. doi: 10.1111/j.1469-0691.2011.03600.x. - DOI - PubMed
-
- Cejas D., Fernandez Canigia L., Nastro M., Rodríguez C., Tanco A., Rodríguez H., Vay C., Maldonado I., Famiglietti A., Giovanakis M., et al. Hyperendemic Clone of KPC producing Klebsiella pneumoniae ST 258 in Buenos Aires hospitals. Infect. Genet. Evol. 2012;12:499–501. doi: 10.1016/j.meegid.2011.09.018. - DOI - PubMed
-
- Cejas D., Elena A., Guevara Nuñez D., Sevillano Platero P., De Paulis A., Magariños F., Alfonso C., Berger M.A., Fernández-Canigia L., Gutkind G., et al. Changing epidemiology of KPC-producing Klebsiella pneumoniae in Argentina: Emergence of hypermucoviscous ST25 and high-risk clone ST307. J. Glob. Antimicrob. Resist. 2019;18:238–242. doi: 10.1016/j.jgar.2019.06.005. - DOI - PubMed
-
- Jure M.A., Castillo M.E., Musa H.E., López C.G., Cáceres M.A., Mochi S.D., Bousquet A.A., Genel N.A., Arlet G.A., Decré D.C. Novel patterns in the molecular epidemiology of KPC-producing Klebsiella pneumoniae in Tucumán, Argentina. J. Glob. Antimicrob. Resist. 2019;19:183–187. doi: 10.1016/j.jgar.2019.02.015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

