Poly(methacrylate citric acid) as a Dual Functional Carrier for Tumor Therapy
- PMID: 36145512
- PMCID: PMC9506429
- DOI: 10.3390/pharmaceutics14091765
Poly(methacrylate citric acid) as a Dual Functional Carrier for Tumor Therapy
Abstract
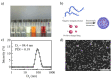
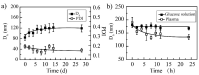
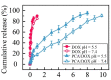
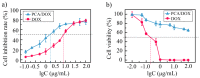
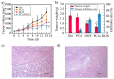
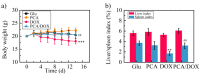
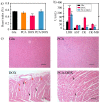
Owing to its pH-sensitive property and chelating Cu2+ effect, poly(methacrylate citric acid) (PCA) can be utilized as a dual functional nanocarrier to construct a nanodelivery system. Negatively charged carboxyl groups can interact with positively charged antineoplastic drugs through electrostatic interaction to form stable drug nanoparticles (NPs). Through drug experimental screening, doxorubicin (DOX) was selected as the model drug, PCA/DOX NPs with a diameter of 84 nm were prepared, and the drug-loading content was 68.3%. PCA/DOX NPs maintained good stability and a sustained release profile. Cell experiments presented that PCA/DOX NPs could inhibit effectively the growth of 4T1 cells; the IC50 value was decreased by approximately 15-fold after incubation for 72 h. The cytotoxicity toward H9C2 was decreased significantly. Moreover, based on its ability to efficiently adsorb copper ions, PCA showed good vascular growth inhibition effect in vitro. Furthermore, animal experiments showed that PCA/DOX NPs presented stronger anticancer effects than DOX; the tumor inhibition rate was increased by 1.5-fold. Myocardial toxicity experiments also confirmed that PCA reduced the cardiotoxicity of DOX. In summary, PCA/DOX NPs show good antitumor efficacy and low toxicity, and have good potential for clinical application.
Keywords: chelating Cu2+ effect; doxorubicin; pH sensitive; poly(methacrylate citric acid).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Hydrophilic Poly(glutamic acid)-Based Nanodrug Delivery System: Structural Influence and Antitumor Efficacy.Polymers (Basel). 2022 May 31;14(11):2242. doi: 10.3390/polym14112242. Polymers (Basel). 2022. PMID: 35683914 Free PMC article.
-
PEGylated Poly(α-lipoic acid) Loaded with Doxorubicin as a pH and Reduction Dual Responsive Nanomedicine for Breast Cancer Therapy.Biomacromolecules. 2018 Nov 12;19(11):4492-4503. doi: 10.1021/acs.biomac.8b01394. Epub 2018 Oct 22. Biomacromolecules. 2018. PMID: 30346147
-
Preparation of doxorubicin-loaded collagen-PAPBA nanoparticles and their anticancer efficacy in ovarian cancer.Ann Transl Med. 2020 Jul;8(14):880. doi: 10.21037/atm-20-5028. Ann Transl Med. 2020. PMID: 32793724 Free PMC article.
-
Improved antitumor activity and reduced toxicity of doxorubicin encapsulated in poly(ε-caprolactone) nanoparticles in lung and breast cancer treatment: An in vitro and in vivo study.Eur J Pharm Sci. 2017 May 1;102:24-34. doi: 10.1016/j.ejps.2017.02.026. Epub 2017 Feb 17. Eur J Pharm Sci. 2017. PMID: 28219748
-
Folate-decorated poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) nanoparticles for targeting delivery: optimization and in vivo antitumor activity.Drug Deliv. 2016 Jun;23(5):1830-7. doi: 10.3109/10717544.2015.1122675. Epub 2015 Dec 11. Drug Deliv. 2016. PMID: 26652055
Cited by
-
Integrating cuproptosis and immunosenescence: A novel therapeutic strategy in cancer treatment.Biochem Biophys Rep. 2025 Mar 27;42:101983. doi: 10.1016/j.bbrep.2025.101983. eCollection 2025 Jun. Biochem Biophys Rep. 2025. PMID: 40224540 Free PMC article. Review.
-
Important functions and molecular mechanisms of aquaporins family on respiratory diseases: potential translational values.J Cancer. 2024 Oct 7;15(18):6073-6085. doi: 10.7150/jca.98829. eCollection 2024. J Cancer. 2024. PMID: 39440058 Free PMC article. Review.
-
Functional Hydrogels and Their Applications in Craniomaxillofacial Bone Regeneration.Pharmaceutics. 2022 Dec 31;15(1):150. doi: 10.3390/pharmaceutics15010150. Pharmaceutics. 2022. PMID: 36678779 Free PMC article. Review.
References
-
- Sionkowska A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011;36:1254–1276. doi: 10.1016/j.progpolymsci.2011.05.003. - DOI
-
- Greiner A.M., Jäckel M., Scheiwe A.C., Stamow D.R., Autenrieth T.J., Lahann J., Franz C.M., Bastmeyer M. Multifunctional polymer scaffolds with adjustable pore size and chemoattractant gradients for studying cell matrix invasion. Biomaterials. 2014;35:611–619. doi: 10.1016/j.biomaterials.2013.09.095. - DOI - PubMed
-
- Gao G., Lange D., Kai H., Kindrachuk J., Zou Y., Cheng J., Kazemzadeh-Narbat M., Kai Y., Wang R., Straus S.K. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials. 2011;32:3899–3909. doi: 10.1016/j.biomaterials.2011.02.013. - DOI - PubMed
-
- Laurencin N. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007;32:762–798.
Grants and funding
LinkOut - more resources
Full Text Sources

