Biopolymeric Prodrug Systems as Potential Antineoplastic Therapy
- PMID: 36145522
- PMCID: PMC9505808
- DOI: 10.3390/pharmaceutics14091773
Biopolymeric Prodrug Systems as Potential Antineoplastic Therapy
Abstract
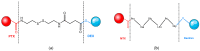
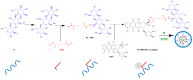
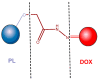
Nowadays, cancer represents a major public health issue, a substantial economic issue, and a burden for society. Limited by numerous disadvantages, conventional chemotherapy is being replaced by new strategies targeting tumor cells. In this context, therapies based on biopolymer prodrug systems represent a promising alternative for improving the pharmacokinetic and pharmacologic properties of drugs and reducing their toxicity. The polymer-directed enzyme prodrug therapy is based on tumor cell targeting and release of the drug using polymer-drug and polymer-enzyme conjugates. In addition, current trends are oriented towards natural sources. They are biocompatible, biodegradable, and represent a valuable and renewable source. Therefore, numerous antitumor molecules have been conjugated with natural polymers. The present manuscript highlights the latest research focused on polymer-drug conjugates containing natural polymers such as chitosan, hyaluronic acid, dextran, pullulan, silk fibroin, heparin, and polysaccharides from Auricularia auricula.
Keywords: Auricularia auricula polysaccharides; PDEPT; antineoplastic therapy; biopolymer; chitosan; dextran; heparin; hyaluronic acid; prodrug systems; pullulan; silk fibroin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Abbas Z., Rehman S. Neoplasm. IntechOpen; London, UK: 2018. An Overview of Cancer Treatment Modalities. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

