Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study
- PMID: 36145605
- PMCID: PMC9505101
- DOI: 10.3390/pharmaceutics14091857
Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study
Abstract
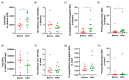
Our previous clinical trial showed that a novel concentrated herbal extract formula, YH1 (Rhizoma coptidis and Shen-Ling-Bai-Zhu-San), improved blood glucose and lipid control. This pilot observational study investigated whether YH1 affects microbiota, plasma, and fecal bile acid (BA) compositions in ten untreated male patients with type 2 diabetes (T2D), hyperlipidemia, and a body mass index ≥ 23 kg/m2. Stool and plasma samples were collected for microbiome, BA, and biochemical analyses before and after 4 weeks of YH1 therapy. As previous studies found, the glycated albumin, 2-h postprandial glucose, triglycerides, total cholesterol, and low-density lipoprotein cholesterol levels were significantly improved after YH1 treatment. Gut microbiota revealed an increased abundance of the short-chain fatty acid-producing bacteria Anaerostipes and Escherichia/Shigella. Furthermore, YH1 inhibited specific phylotypes of bile salt hydrolase-expressing bacteria, including Parabacteroides, Bifidobacterium, and Bacteroides caccae. Stool tauro-conjugated BA levels increased after YH1 treatment. Plasma total BAs and 7α-hydroxy-4-cholesten-3-one (C4), a BA synthesis indicator, were elevated. The reduced deconjugation of BAs and increased plasma conjugated BAs, especially tauro-conjugated BAs, led to a decreased glyco- to tauro-conjugated BA ratio and reduced unconjugated secondary BAs. These results suggest that YH1 ameliorates T2D and hyperlipidemia by modulating microbiota constituents that alter fecal and plasma BA compositions and promote liver cholesterol-to-BA conversion and glucose homeostasis.
Keywords: Chinese herbal medicine; YH1; bile acids; gut microbiota; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Huang Y.H., Chen S.T., Liu F.H., Hsieh S.H., Lin C.H., Liou M.J., Wang C.C., Huang C.H., Liu G.H., Lin J.R., et al. The efficacy and safety of concentrated herbal extract granules, YH1, as an add-on medication in poorly controlled type 2 diabetes: A randomized, double-blind, placebo-controlled pilot trial. PLoS ONE. 2019;14:e0221199. doi: 10.1371/journal.pone.0221199. - DOI - PMC - PubMed
-
- Yin J., Ye J., Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B. 2012;2:327–334. doi: 10.1016/j.apsb.2012.06.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

