Lactoferrin-Conjugated Nanoparticles as New Antivirals
- PMID: 36145610
- PMCID: PMC9504495
- DOI: 10.3390/pharmaceutics14091862
Lactoferrin-Conjugated Nanoparticles as New Antivirals
Abstract
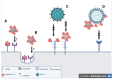
Lactoferrin is an iron-binding glycoprotein with multiple functions in the body. Its activity against a broad spectrum of both DNA and RNA viruses as well as the ability to modulate immune responses have made it of interest in the pharmaceutical and food industries. The mechanisms of its antiviral activity include direct binding to the viruses or its receptors or the upregulation of antiviral responses by the immune system. Recently, much effort has been devoted to the use of nanotechnology in the development of new antivirals. In this review, we focus on describing the antiviral mechanisms of lactoferrin and the possible use of nanotechnology to construct safe and effective new antiviral drugs.
Keywords: lactoferrin; nanoparticles; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

