Novel 3D-Printed Dressings of Chitosan-Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis
- PMID: 36145714
- PMCID: PMC9503579
- DOI: 10.3390/pharmaceutics14091966
Novel 3D-Printed Dressings of Chitosan-Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis
Abstract
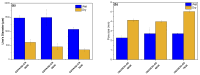
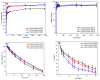
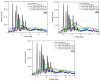
In the present study, the blends of CS and Vanillin-CS derivative (VACS) were utilized for the preparation of printable inks for their application in three-dimensional (3D) printing procedures. Despite the synergic interaction between the blends, the addition of ι-carrageenan (iCR) as a thickening agent was mandatory. Their viscosity analysis was conducted for the evaluation of the optimum CS/VACS ratio. The shear thinning behavior along with the effect of the temperature on viscosity values were evident. Further characterization of the 3D-printed structures was conducted. The effect of the CS/VACS ratio was established through swelling and contact angle measurements. An increasing amount of VACS resulted in lower swelling ability along with higher hydrophobicity. Fluticasone propionate (FLU), a crystalline synthetic corticosteroid, was loaded into the CS/VACS samples. The drug was loaded in its amorphous state, and consequently, its in vitro release was significantly enhanced. An initial burst release, followed by a sustained release profile, was observed.
Keywords: 3D printing; atopic dermatitis; chitosan; drug release; fluticasone propionate; polymer blends.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Exploring the Blends' Miscibility of a Novel Chitosan Derivative with Enhanced Antioxidant Properties; Prospects for 3D Printing Biomedical Applications.Mar Drugs. 2023 Jun 22;21(7):370. doi: 10.3390/md21070370. Mar Drugs. 2023. PMID: 37504901 Free PMC article.
-
Vanillin chitosan miscible hydrogel blends and their prospects for 3D printing biomedical applications.Int J Biol Macromol. 2021 Dec 1;192:1266-1275. doi: 10.1016/j.ijbiomac.2021.10.093. Epub 2021 Oct 20. Int J Biol Macromol. 2021. PMID: 34687759
-
Preliminary Evaluation of 3D Printed Chitosan/Pectin Constructs for Biomedical Applications.Mar Drugs. 2021 Jan 15;19(1):36. doi: 10.3390/md19010036. Mar Drugs. 2021. PMID: 33467462 Free PMC article.
-
Chitosan and Whey Protein Bio-Inks for 3D and 4D Printing Applications with Particular Focus on Food Industry.Molecules. 2021 Dec 28;27(1):173. doi: 10.3390/molecules27010173. Molecules. 2021. PMID: 35011406 Free PMC article. Review.
-
Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends.Materials (Basel). 2020 Jun 11;13(11):2663. doi: 10.3390/ma13112663. Materials (Basel). 2020. PMID: 32545256 Free PMC article. Review.
Cited by
-
Exploring the Blends' Miscibility of a Novel Chitosan Derivative with Enhanced Antioxidant Properties; Prospects for 3D Printing Biomedical Applications.Mar Drugs. 2023 Jun 22;21(7):370. doi: 10.3390/md21070370. Mar Drugs. 2023. PMID: 37504901 Free PMC article.
-
Synthesis and Evaluation of Poly(3-hydroxypropyl Ethylene-imine) and Its Blends with Chitosan Forming Novel Elastic Films for Delivery of Haloperidol.Pharmaceutics. 2022 Nov 30;14(12):2671. doi: 10.3390/pharmaceutics14122671. Pharmaceutics. 2022. PMID: 36559165 Free PMC article.
References
-
- Zaman M., Khalid U., Abdul M., Raja G., Sultana K., Amjad M.W., Rehman A.U. Fabrication and Characterization of Matrix Type Transdermal Patches Loaded with Ramipril and Repaglinide via Cellulose Based Hydrophilic and Hydrophobic Polymers; In-vitro and Ex-vivo Permeation Studies. Polym. Plast. Technol. Eng. 2017;56:1713–1722. doi: 10.1080/03602559.2017.1289400. - DOI
-
- Doozandeh Z., Saber-samandari S., Khandan A. Preparation of Novel Arabic Gum-C6H9NO Biopolymer as a Bedsore for Wound Care Application. Acta Med. Iran. 2020;58:520–530. doi: 10.18502/acta.v58i10.4915. - DOI
-
- Rahmani M., Bidgoli S.A., Rezayat M. Electrospun polymeric nanofibers for transdermal drug delivery. Nanomed. J. 2017;4:61–70. doi: 10.22038/nmj.2017.8407. - DOI
LinkOut - more resources
Full Text Sources

