Advancement in the Breeding, Biotechnological and Genomic Tools towards Development of Durable Genetic Resistance against the Rice Blast Disease
- PMID: 36145787
- PMCID: PMC9504543
- DOI: 10.3390/plants11182386
Advancement in the Breeding, Biotechnological and Genomic Tools towards Development of Durable Genetic Resistance against the Rice Blast Disease
Abstract
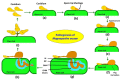
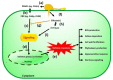
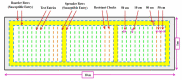
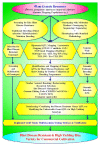
Rice production needs to be sustained in the coming decades, as the changeable climatic conditions are becoming more conducive to disease outbreaks. The majority of rice diseases cause enormous economic damage and yield instability. Among them, rice blast caused by Magnaportheoryzae is a serious fungal disease and is considered one of the major threats to world rice production. This pathogen can infect the above-ground tissues of rice plants at any growth stage and causes complete crop failure under favorable conditions. Therefore, management of blast disease is essentially required to sustain global food production. When looking at the drawback of chemical management strategy, the development of durable, resistant varieties is one of the most sustainable, economic, and environment-friendly approaches to counter the outbreaks of rice blasts. Interestingly, several blast-resistant rice cultivars have been developed with the help of breeding and biotechnological methods. In addition, 146 R genes have been identified, and 37 among them have been molecularly characterized to date. Further, more than 500 loci have been identified for blast resistance which enhances the resources for developing blast resistance through marker-assisted selection (MAS), marker-assisted backcross breeding (MABB), and genome editing tools. Apart from these, a better understanding of rice blast pathogens, the infection process of the pathogen, and the genetics of the immune response of the host plant are very important for the effective management of the blast disease. Further, high throughput phenotyping and disease screening protocols have played significant roles in easy comprehension of the mechanism of disease spread. The present review critically emphasizes the pathogenesis, pathogenomics, screening techniques, traditional and molecular breeding approaches, and transgenic and genome editing tools to develop a broad spectrum and durable resistance against blast disease in rice. The updated and comprehensive information presented in this review would be definitely helpful for the researchers, breeders, and students in the planning and execution of a resistance breeding program in rice against this pathogen.
Keywords: Magnaporthe oryzae; R-genes; blast disease; broad-spectrum resistance; conventional breeding; genome editing; genomic tools; molecular breeding; rice.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Blast resistance in rice: a review of conventional breeding to molecular approaches.Mol Biol Rep. 2013 Mar;40(3):2369-88. doi: 10.1007/s11033-012-2318-0. Epub 2012 Nov 27. Mol Biol Rep. 2013. PMID: 23184051 Review.
-
Molecular Breeding Strategy and Challenges Towards Improvement of Blast Disease Resistance in Rice Crop.Front Plant Sci. 2015 Nov 16;6:886. doi: 10.3389/fpls.2015.00886. eCollection 2015. Front Plant Sci. 2015. PMID: 26635817 Free PMC article. Review.
-
Marker Assisted Introgression of Resistance Genes and Phenotypic Evaluation Enabled Identification of Durable and Broad-Spectrum Blast Resistance in Elite Rice Cultivar, CO 51.Genes (Basel). 2023 Mar 15;14(3):719. doi: 10.3390/genes14030719. Genes (Basel). 2023. PMID: 36980991 Free PMC article.
-
Status on Genetic Resistance to Rice Blast Disease in the Post-Genomic Era.Plants (Basel). 2025 Mar 5;14(5):807. doi: 10.3390/plants14050807. Plants (Basel). 2025. PMID: 40094775 Free PMC article. Review.
-
Current advance methods for the identification of blast resistance genes in rice.C R Biol. 2015 May;338(5):321-34. doi: 10.1016/j.crvi.2015.03.001. Epub 2015 Apr 2. C R Biol. 2015. PMID: 25843222
Cited by
-
Pathogenicity Analyses of Rice Blast Fungus (Pyricularia oryzae) from Japonica Rice Area of Northeast China.Pathogens. 2024 Feb 28;13(3):211. doi: 10.3390/pathogens13030211. Pathogens. 2024. PMID: 38535554 Free PMC article.
-
Development and Application of Pik Locus-Specific Molecular Markers for Blast Resistance Genes in Yunnan Japonica Rice Cultivars.Plants (Basel). 2025 Feb 15;14(4):592. doi: 10.3390/plants14040592. Plants (Basel). 2025. PMID: 40006851 Free PMC article.
-
Allelic variation in rice blast resistance: a pathway to sustainable disease management.Mol Biol Rep. 2024 Aug 24;51(1):935. doi: 10.1007/s11033-024-09854-2. Mol Biol Rep. 2024. PMID: 39180629 Review.
-
Rice Varieties Intercropping Induced Soil Metabolic and Microbial Recruiting to Enhance the Rice Blast (Magnaporthe Oryzae) Resistance.Metabolites. 2024 Sep 20;14(9):507. doi: 10.3390/metabo14090507. Metabolites. 2024. PMID: 39330514 Free PMC article.
-
Genetic Improvement in Plant Architecture, Maturity Duration and Agronomic Traits of Three Traditional Rice Landraces through Gamma Ray-Based Induced Mutagenesis.Plants (Basel). 2022 Dec 9;11(24):3448. doi: 10.3390/plants11243448. Plants (Basel). 2022. PMID: 36559562 Free PMC article.
References
-
- Simkhada K., Thapa R. Rice Blast, A Major Threat to the Rice Production and Its Various Management Techniques. Turkish J. Agric. Food Sci. Technol. 2022;10:147–157. doi: 10.24925/turjaf.v10i2.147-157.4548. - DOI
-
- Asibi E.A., Chai Q., Coulter J.A. Rice Blast: A Disease with Implications for Global Food Security. Agronomy. 2019;9:451. doi: 10.3390/agronomy9080451. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

