Rhizobacteria Mitigate the Negative Effect of Aluminum on Pea Growth by Immobilizing the Toxicant and Modulating Root Exudation
- PMID: 36145816
- PMCID: PMC9503566
- DOI: 10.3390/plants11182416
Rhizobacteria Mitigate the Negative Effect of Aluminum on Pea Growth by Immobilizing the Toxicant and Modulating Root Exudation
Abstract
High soil acidity is one of the main unfavorable soil factors that inhibit the growth and mineral nutrition of plants. This is largely due to the toxicity of aluminum (Al), the mobility of which increases significantly in acidic soils. Symbiotic microorganisms have a wide range of beneficial properties for plants, protecting them against abiotic stress factors. This report describes the mechanisms of positive effects of plant growth-promoting rhizobacteria Pseudomonas fluorescens SPB2137 on four pea (Pisum sativum L.) genotypes grown in hydroponics and treated with 80 µM AlCl3. In batch culture, the bacteria produced auxins, possessed 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity, alkalized the medium and immobilized Al, forming biofilm-like structures and insoluble phosphates. Inoculation with Ps. fluorescens SPB2137 increased root and/or shoot biomass of Al-treated plants. The bacteria alkalized the nutrient solution and transferred Al from the solution to the residue, which contained phosphorus that was exuded by roots. As a result, the Al concentration in roots decreased, while the amount of precipitated Al correlated negatively with its concentration in the solution, positively with the solution pH and negatively with Al concentration in roots and shoots. Treatment with Al induced root exudation of organic acids, amino acids and sugars. The bacteria modulated root exudation via utilization and/or stimulation processes. The effects of Al and bacteria on plants varied depending on pea genotype, but all the effects had a positive direction and the variability was mostly quantitative. Thus, Ps. fluorescens SPB2137 improved the Al tolerance of pea due to immobilization and exclusion of toxicants from the root zone.
Keywords: PGPR; aluminum; immobilization; pea; phosphorus; rhizosphere; root exudation; soil acidity; symbiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

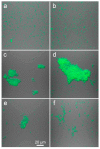
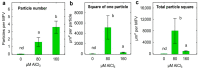

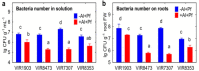


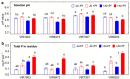

Similar articles
-
Aluminum-Immobilizing Rhizobacteria Modulate Root Exudation and Nutrient Uptake and Increase Aluminum Tolerance of Pea Mutant E107 (brz).Plants (Basel). 2023 Jun 15;12(12):2334. doi: 10.3390/plants12122334. Plants (Basel). 2023. PMID: 37375958 Free PMC article.
-
Aluminum exclusion from root zone and maintenance of nutrient uptake are principal mechanisms of Al tolerance in Pisum sativum L.Physiol Mol Biol Plants. 2017 Oct;23(4):851-863. doi: 10.1007/s12298-017-0469-0. Epub 2017 Sep 18. Physiol Mol Biol Plants. 2017. PMID: 29158634 Free PMC article.
-
The Role of Symbiotic Microorganisms, Nutrient Uptake and Rhizosphere Bacterial Community in Response of Pea (Pisum sativum L.) Genotypes to Elevated Al Concentrations in Soil.Plants (Basel). 2020 Dec 18;9(12):1801. doi: 10.3390/plants9121801. Plants (Basel). 2020. PMID: 33353122 Free PMC article.
-
Possible mechanisms for the equilibrium of ACC and role of ACC deaminase-producing bacteria.Appl Microbiol Biotechnol. 2022 Feb;106(3):877-887. doi: 10.1007/s00253-022-11772-x. Epub 2022 Jan 21. Appl Microbiol Biotechnol. 2022. PMID: 35061091 Review.
-
Aluminum phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies.Ecotoxicol Environ Saf. 2024 Jan 1;269:115791. doi: 10.1016/j.ecoenv.2023.115791. Epub 2023 Dec 8. Ecotoxicol Environ Saf. 2024. PMID: 38070417 Review.
Cited by
-
Draft Genome Sequence of the Bacterium Cupriavidus sp. Strain D39, Inhabiting the Rhizosphere of Pea Plants (Pisum sativum L.).Microbiol Resour Announc. 2023 Apr 18;12(4):e0135422. doi: 10.1128/mra.01354-22. Epub 2023 Mar 21. Microbiol Resour Announc. 2023. PMID: 36943044 Free PMC article.
-
Aluminum-Immobilizing Rhizobacteria Modulate Root Exudation and Nutrient Uptake and Increase Aluminum Tolerance of Pea Mutant E107 (brz).Plants (Basel). 2023 Jun 15;12(12):2334. doi: 10.3390/plants12122334. Plants (Basel). 2023. PMID: 37375958 Free PMC article.
-
Deciphering the differences of bacterial communities between high- and low-productive wheat fields using high-throughput sequencing.Front Microbiol. 2024 Sep 4;15:1391428. doi: 10.3389/fmicb.2024.1391428. eCollection 2024. Front Microbiol. 2024. PMID: 39296300 Free PMC article.
-
Abscisic Acid Metabolizing Rhodococcus sp. Counteracts Phytopathogenic Effects of Abscisic Acid Producing Botrytis sp. on Sunflower Seedlings.Plants (Basel). 2025 Aug 7;14(15):2442. doi: 10.3390/plants14152442. Plants (Basel). 2025. PMID: 40805791 Free PMC article.
References
-
- Taylor G.J. Current views of the aluminum stress response: The physiological basis of tolerance. Plant Physiol. Biochem. 1991;10:57–93.
-
- Gupta N., Gaurav S.S., Kumar A. Molecular basis of aluminium toxicity in plants: A review. Am. J. Plant. Sci. 2013;4:21–37. doi: 10.4236/ajps.2013.412A3004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

