Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration
- PMID: 36146238
- PMCID: PMC9503911
- DOI: 10.3390/s22186889
Kidney-on-a-Chip: Mechanical Stimulation and Sensor Integration
Abstract
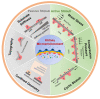
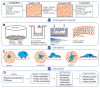
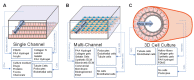
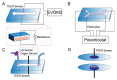
Bioengineered in vitro models of the kidney offer unprecedented opportunities to better mimic the in vivo microenvironment. Kidney-on-a-chip technology reproduces 2D or 3D features which can replicate features of the tissue architecture, composition, and dynamic mechanical forces experienced by cells in vivo. Kidney cells are exposed to mechanical stimuli such as substrate stiffness, shear stress, compression, and stretch, which regulate multiple cellular functions. Incorporating mechanical stimuli in kidney-on-a-chip is critically important for recapitulating the physiological or pathological microenvironment. This review will explore approaches to applying mechanical stimuli to different cell types using kidney-on-a-chip models and how these systems are used to study kidney physiology, model disease, and screen for drug toxicity. We further discuss sensor integration into kidney-on-a-chip for monitoring cellular responses to mechanical or other pathological stimuli. We discuss the advantages, limitations, and challenges associated with incorporating mechanical stimuli in kidney-on-a-chip models for a variety of applications. Overall, this review aims to highlight the importance of mechanical stimuli and sensor integration in the design and implementation of kidney-on-a-chip devices.
Keywords: glomerulus; kidney-on-a-chip; mechanical stimuli; microfluidic; proximal tubule; shear stress; substrate stiffness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Small Force, Big Impact: Next Generation Organ-on-a-Chip Systems Incorporating Biomechanical Cues.Front Physiol. 2018 Oct 9;9:1417. doi: 10.3389/fphys.2018.01417. eCollection 2018. Front Physiol. 2018. PMID: 30356887 Free PMC article. Review.
-
Kidney-on-a-chip: untapped opportunities.Kidney Int. 2018 Dec;94(6):1073-1086. doi: 10.1016/j.kint.2018.06.034. Epub 2018 Oct 23. Kidney Int. 2018. PMID: 30366681 Free PMC article. Review.
-
Integrated microfluidic chip for endothelial cells culture and analysis exposed to a pulsatile and oscillatory shear stress.Lab Chip. 2009 Nov 7;9(21):3118-25. doi: 10.1039/b909312e. Epub 2009 Aug 18. Lab Chip. 2009. PMID: 19823728
-
Tumor-on-a-chip for integrating a 3D tumor microenvironment: chemical and mechanical factors.Lab Chip. 2020 Mar 3;20(5):873-888. doi: 10.1039/c9lc00550a. Lab Chip. 2020. PMID: 32025687 Free PMC article. Review.
-
Application of physiological shear stress to renal tubular epithelial cells.Methods Cell Biol. 2019;153:43-67. doi: 10.1016/bs.mcb.2019.04.010. Epub 2019 May 24. Methods Cell Biol. 2019. PMID: 31395384
Cited by
-
Open-Source System for Real-Time Functional Assessment of In Vitro Filtration Barriers.Ann Biomed Eng. 2024 Feb;52(2):327-341. doi: 10.1007/s10439-023-03378-9. Epub 2023 Oct 29. Ann Biomed Eng. 2024. PMID: 37899379 Free PMC article.
-
Advanced In Vitro Models for Preclinical Drug Safety: Recent Progress and Prospects.Curr Issues Mol Biol. 2024 Dec 26;47(1):7. doi: 10.3390/cimb47010007. Curr Issues Mol Biol. 2024. PMID: 39852122 Free PMC article. Review.
-
Effective and new technologies in kidney tissue engineering.Front Bioeng Biotechnol. 2024 Oct 16;12:1476510. doi: 10.3389/fbioe.2024.1476510. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39479295 Free PMC article. Review.
-
An open-source, battery-powered, low-cost, and dual-channel pneumatic pulse generator for microfluidic cell-stretch assays.HardwareX. 2024 Oct 11;20:e00595. doi: 10.1016/j.ohx.2024.e00595. eCollection 2024 Dec. HardwareX. 2024. PMID: 39483396 Free PMC article.
-
Integrating tumor and healthy epithelium in a micro-physiology multi-compartment approach to study renal cell carcinoma pathophysiology.Sci Rep. 2024 Apr 23;14(1):9357. doi: 10.1038/s41598-024-60164-w. Sci Rep. 2024. PMID: 38653823 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

