RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp
- PMID: 36146509
- PMCID: PMC9504209
- DOI: 10.3390/vaccines10091428
RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp
Abstract
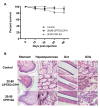
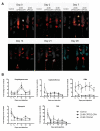
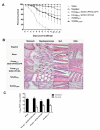
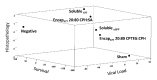
In the last 15 years, crustacean fisheries have experienced billions of dollars in economic losses, primarily due to viral diseases caused by such pathogens as white spot syndrome virus (WSSV) in the Pacific white shrimp Litopenaeus vannamei and Asian tiger shrimp Penaeus monodon. To date, no effective measures are available to prevent or control disease outbreaks in these animals, despite their economic importance. Recently, double-stranded RNA-based vaccines have been shown to provide specific and robust protection against WSSV infection in cultured shrimp. However, the limited stability of double-stranded RNA is the most significant hurdle for the field application of these vaccines with respect to delivery within an aquatic system. Polyanhydride nanoparticles have been successfully used for the encapsulation and release of vaccine antigens. We have developed a double-stranded RNA-based nanovaccine for use in shrimp disease control with emphasis on the Pacific white shrimp L. vannamei. Nanoparticles based on copolymers of sebacic acid, 1,6-bis(p-carboxyphenoxy)hexane, and 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane exhibited excellent safety profiles, as measured by shrimp survival and histological evaluation. Furthermore, the nanoparticles localized to tissue target replication sites for WSSV and persisted through 28 days postadministration. Finally, the nanovaccine provided ~80% protection in a lethal WSSV challenge model. This study demonstrates the exciting potential of a safe, effective, and field-applicable RNA nanovaccine that can be rationally designed against infectious diseases affecting aquaculture.
Keywords: WSSV; dsRNA; nanovaccine; polyanhydride; shrimp.
Conflict of interest statement
Y.P. has financial interests in Pan Genome Systems, a company developing animal and human vaccines. Balaji Narasimhan is a cofounder of ImmunoNanoMed, a start-up with business interests in the development of nanobased vaccines against infectious diseases. Narasimhan also has a financial interest in Degimflex, a start-up with business interests in the development of flexible degradable electronic films for biomedical applications.
Figures


References
-
- Food and Agricultural Organization of the United Nations Fisheries and Aquaculture Department, FishStat Plus-Fishery Statistical Software. 2009. [(accessed on 16 August 2022)]. Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj/en.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

