Predicting SARS-CoV-2 Variant Spread in a Completely Seropositive Population Using Semi-Quantitative Antibody Measurements in Blood Donors
- PMID: 36146515
- PMCID: PMC9501043
- DOI: 10.3390/vaccines10091437
Predicting SARS-CoV-2 Variant Spread in a Completely Seropositive Population Using Semi-Quantitative Antibody Measurements in Blood Donors
Abstract
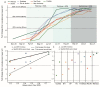
SARS-CoV-2 serologic surveys estimate the proportion of the population with antibodies against historical variants, which nears 100% in many settings. New approaches are required to fully exploit serosurvey data. Using a SARS-CoV-2 anti-Spike (S) protein chemiluminescent microparticle assay, we attained a semi-quantitative measurement of population IgG titers in serial cross-sectional monthly samples of blood donations across seven Brazilian state capitals (March 2021−November 2021). Using an ecological analysis, we assessed the contributions of prior attack rate and vaccination to antibody titer. We compared anti-S titer across the seven cities during the growth phase of the Delta variant and used this to predict the resulting age-standardized incidence of severe COVID-19 cases. We tested ~780 samples per month, per location. Seroprevalence rose to >95% across all seven capitals by November 2021. Driven by vaccination, mean antibody titer increased 16-fold over the study, with the greatest increases occurring in cities with the highest prior attack rates. Mean anti-S IgG was strongly correlated (adjusted R2 = 0.89) with the number of severe cases caused by Delta. Semi-quantitative anti-S antibody titers are informative about prior exposure and vaccination coverage and may also indicate the potential impact of future SARS-CoV-2 variants.
Keywords: SARS-CoV-2; delta; immunity; seroprevalence; vaccines; variants of concern.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Bingham J., Cable R., Coleman C., Glatt T.N., Grebe E., Mhlanga L., Nyano C., Pieterson N., Swanevelder R., Swarts A., et al. Estimates of Prevalence of Anti-SARS-CoV-2 Antibodies among Blood Donors in South Africa in March 2022. [(accessed on 30 May 2022)]. Available online: - DOI
-
- CDC Coronavirus Disease 2019 (COVID-19) [(accessed on 25 May 2022)];2020 Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classificatio....
-
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7. in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. - DOI - PMC - PubMed
-
- Allen H., Vusirikala A., Flannagan J., Twohig K.A., Zaidi A., Chudasama D., Lamagni T., Groves N., Turner C., Rawlinson C., et al. Household transmission of COVID-19 cases associated with SARS-CoV-2 delta variant (B.1.617.2): National case-control study. Lancet Reg. Health Eur. 2022;12:100252. doi: 10.1016/j.lanepe.2021.100252. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

