Influenza B Virus (IBV) Immune-Mediated Disease in C57BL/6 Mice
- PMID: 36146518
- PMCID: PMC9504307
- DOI: 10.3390/vaccines10091440
Influenza B Virus (IBV) Immune-Mediated Disease in C57BL/6 Mice
Abstract
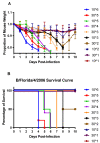
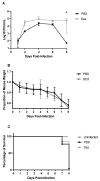
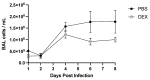
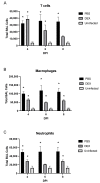
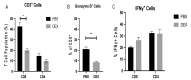
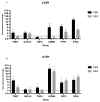
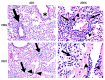
Influenza B viruses (IBV) primarily infect humans, causing seasonal epidemics. The absence of an animal reservoir limits pandemic concern, but IBV infections may cause severe respiratory disease, predominantly in young children and the elderly. The IBV disease burden is largely controlled by seasonal influenza vaccination; however, immunity due to vaccination is sometimes incomplete, a feature linked to antigenic mismatches. Thus, understanding the features that contribute to disease pathogenesis is important, particularly immune-mediated versus virus-mediated outcomes. Unexpectedly, C57BL/6 (B6) mice intranasally infected with a low multiplicity of infection of B/Florida/04/2006 developed substantial morbidity and mortality. To address the cause, B6 mice were treated daily with dexamethasone to dampen the immune and pro-inflammatory response to IBV infection, allowing the determination of whether the responses were immune- and/or virus-associated. As expected, dexamethasone (DEX)-treated mice had a lower pro-inflammatory response and reduced lung pathology despite the presence of high viral lung titers, but mortality was comparable to PBS-treated mice, indicating that mortality may be linked to lung virus replication. The results showed that the immune response to IBV is the major cause of morbidity, mortality, lung pathology, and viral clearance. Importantly, the results suggest that a robust lung CTL response and associated leukocyte influx contribute to disease.
Keywords: IBV; dexamethasone; host–pathogen interaction; immune-mediated disease; influenza B virus; mouse model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Domestic pigs are susceptible to infection with influenza B viruses.J Virol. 2015 May;89(9):4818-26. doi: 10.1128/JVI.00059-15. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673727 Free PMC article.
-
Development of an Alternative Modified Live Influenza B Virus Vaccine.J Virol. 2017 May 26;91(12):e00056-17. doi: 10.1128/JVI.00056-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28381580 Free PMC article.
-
Modeling the effects of cigarette smoke extract on influenza B virus infections in mice.Front Immunol. 2023 Mar 23;14:1083251. doi: 10.3389/fimmu.2023.1083251. eCollection 2023. Front Immunol. 2023. PMID: 37033954 Free PMC article.
-
Innate and adaptive immunity toward influenza B viruses.Future Microbiol. 2020 Jul;15:1045-1058. doi: 10.2217/fmb-2019-0340. Epub 2020 Aug 19. Future Microbiol. 2020. PMID: 32811172 Review.
-
Knowns and unknowns of influenza B viruses.Future Microbiol. 2016;11(1):119-35. doi: 10.2217/fmb.15.120. Epub 2015 Dec 18. Future Microbiol. 2016. PMID: 26684590 Review.
Cited by
-
Immune Prophylaxis Targeting the Respiratory Syncytial Virus (RSV) G Protein.Viruses. 2023 Apr 27;15(5):1067. doi: 10.3390/v15051067. Viruses. 2023. PMID: 37243153 Free PMC article.
-
Development of an AlphaLISA assay for sensitive and accurate detection of influenza B virus.Front Med (Lausanne). 2023 May 5;10:1155551. doi: 10.3389/fmed.2023.1155551. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37215702 Free PMC article.
-
Nanoparticles and Antiviral Vaccines.Vaccines (Basel). 2023 Dec 27;12(1):30. doi: 10.3390/vaccines12010030. Vaccines (Basel). 2023. PMID: 38250843 Free PMC article. Review.
References
-
- Caini S., Kusznierz G., Garate V.V., Wangchuk S., Thapa B., Júnior F.J.D.P., De Almeida W.A.F., Njouom R., Fasce R.A., Bustos P., et al. The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS ONE. 2019;14:e0222381. doi: 10.1371/journal.pone.0222381. - DOI - PMC - PubMed
-
- Gutiérrez-Pizarraya A., Pérez-Romero P., Alvarez R., Aydillo T., Osorio-Gómez G., Milara-Ibáñez C., Sánchez M., Pachón J., Cordero E. Unexpected severity of cases of influenza B infection in patients that required hospitalization during the first postpandemic wave. J. Infect. 2012;65:423–430. doi: 10.1016/j.jinf.2012.07.004. - DOI - PubMed
-
- Su S., Chaves S.S., Perez A., D’Mello T., Kirley P.D., Yousey-Hindes K., Farley M.M., Harris M., Sharangpani R., Lynfield R., et al. Comparing Clinical Characteristics Between Hospitalized Adults With Laboratory-Confirmed Influenza A and B Virus Infection. Clin. Infect. Dis. 2014;59:252–255. doi: 10.1093/cid/ciu269. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

