Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine over Three Consecutive Influenza Seasons in the United States
- PMID: 36146534
- PMCID: PMC9504704
- DOI: 10.3390/vaccines10091456
Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine over Three Consecutive Influenza Seasons in the United States
Abstract
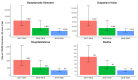
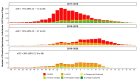
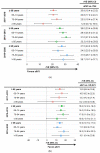
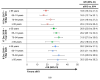
Traditional influenza vaccines may be less immunogenic in adults ≥65 years of age due to immunosenescence. Two influenza vaccines-MF59®-adjuvanted trivalent inactivated influenza vaccine (aIIV3) and high-dose influenza vaccine (HD-IIV3)-were developed to overcome this problem. We summarize estimates of the relative vaccine effectiveness (rVE) of aIIV3 vs. HD-IIV3 and aIIV3 vs. standard, egg-based quadrivalent influenza vaccines (IIV4e) during the 2017-2018, 2018-2019, and 2019-2020 US influenza seasons using the same underlying electronic medical record and linked claims dataset for all three seasons. The primary outcome was influenza-related medical encounters (IRMEs), defined by diagnostic codes specific to influenza (ICD J09*-J11*). rVE was estimated using propensity score methods adjusting for demographics and health status. rVE estimates demonstrated consistent benefit for aIIV3 over IIV4e in the overall and at-risk populations. Relative to HD-IIV3, aIIV3 provided improved benefit in the overall study population and comparable benefit in the at-risk population across each season.
Keywords: immunosenescence; influenza; influenza vaccines; relative vaccine effectiveness.
Conflict of interest statement
C. Boikos, I. McGovern, E. Versage and M. Haag are employees of Seqirus, and this work was funded by Seqirus. J.R. Ortiz reports grants to his institution from NIH for influenza vaccine research, grants to his institution from Pfizer for non-influenza vaccine research, and honoraria from Seqirus to serve on the Real World Evidence Scientific Advisory Board, from Pfizer to serve on the Immunization for All Ages Advisory Board, and from Moderna to serve on the New Vaccines Scientific Advisory Board. J. Puig-Barberà reports honoraria for academic activities from Sanofi Pasteur and Seqirus, Advisory board membership from Seqirus, and research funding from Seqirus.
Figures

Similar articles
-
Relative Effectiveness of Adjuvanted Trivalent Inactivated Influenza Vaccine Versus Egg-derived Quadrivalent Inactivated Influenza Vaccines and High-dose Trivalent Influenza Vaccine in Preventing Influenza-related Medical Encounters in US Adults ≥ 65 Years During the 2017-2018 and 2018-2019 Influenza Seasons.Clin Infect Dis. 2021 Sep 7;73(5):816-823. doi: 10.1093/cid/ciab152. Clin Infect Dis. 2021. PMID: 33605977 Free PMC article.
-
Relative Effectiveness of MF59 Adjuvanted Trivalent Influenza Vaccine vs Nonadjuvanted Vaccines During the 2019-2020 Influenza Season.Open Forum Infect Dis. 2022 Apr 2;9(5):ofac167. doi: 10.1093/ofid/ofac167. eCollection 2022 May. Open Forum Infect Dis. 2022. PMID: 35493131 Free PMC article.
-
Relative Effectiveness of the MF59®-Adjuvanted Influenza Vaccine Versus High-Dose and Non-Adjuvanted Influenza Vaccines in Preventing Cardiorespiratory Hospitalizations During the 2019-2020 US Influenza Season.Influenza Other Respir Viruses. 2024 Apr;18(4):e13288. doi: 10.1111/irv.13288. Influenza Other Respir Viruses. 2024. PMID: 38644564 Free PMC article.
-
Fluzone® High-Dose Influenza Vaccine.Expert Rev Vaccines. 2016 Dec;15(12):1495-1505. doi: 10.1080/14760584.2016.1254044. Epub 2016 Nov 14. Expert Rev Vaccines. 2016. PMID: 27813430 Review.
-
Monitoring the safety of high-dose, trivalent inactivated influenza vaccine in the vaccine adverse event reporting system (VAERS), 2011 - 2019.Vaccine. 2020 Aug 18;38(37):5923-5926. doi: 10.1016/j.vaccine.2020.07.007. Epub 2020 Jul 21. Vaccine. 2020. PMID: 32709434 Review.
Cited by
-
[Enhanced targeted influenza vaccines - New evidence shows higher effectiveness in older adults].Dtsch Med Wochenschr. 2023 Apr;148(9):556-562. doi: 10.1055/a-2032-1368. Epub 2023 Mar 29. Dtsch Med Wochenschr. 2023. PMID: 36990440 Free PMC article. German.
-
Rollout of the 2022/2023 Seasonal Influenza Vaccination and Correlates of the Use of Enhanced Vaccines among Italian Adults.Vaccines (Basel). 2023 Nov 23;11(12):1748. doi: 10.3390/vaccines11121748. Vaccines (Basel). 2023. PMID: 38140153 Free PMC article.
-
Influenza disease burden and the need for highly immunogenic vaccines in older adults: a narrative review.Ewha Med J. 2024 Jul;47(3):e35. doi: 10.12771/emj.2024.e35. Epub 2024 Jul 31. Ewha Med J. 2024. PMID: 40703460 Free PMC article. Review.
References
-
- Izurieta H.S., Chillarige Y., Kelman J., Wei Y., Lu Y., Xu W., Lu M., Pratt D., Chu S., Wernecke M., et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J. Infect. Dis. 2019;220:1255–1264. doi: 10.1093/infdis/jiy716. - DOI - PubMed
-
- Centers for Disease Control and Prevention CDC Vaccine Effectiveness Networks. [(accessed on 7 October 2021)]; Available online: https://www.cdc.gov/flu/vaccines-work/vaccine-effectiveness-networks.htm.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

