Longitudinal Comparison of Neutralizing Antibody Responses to COVID-19 mRNA Vaccines after Second and Third Doses
- PMID: 36146537
- PMCID: PMC9504054
- DOI: 10.3390/vaccines10091459
Longitudinal Comparison of Neutralizing Antibody Responses to COVID-19 mRNA Vaccines after Second and Third Doses
Abstract
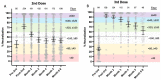
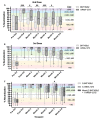
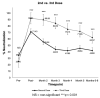
COVID-19 mRNA vaccines protect against severe disease and hospitalization. Neutralizing antibodies (NAbs) are a first-line defense mechanism, but protective NAb responses are variable. Currently, NAb testing is not widely available. This study employed a lateral flow assay for monitoring NAb levels postvaccination and natural infection, using a finger-stick drop of blood. We report longitudinal NAb data from BNT162b2 (Pfizer) and mRNA-1273 (Moderna) recipients after second and third doses. Results demonstrate a third dose of mRNA vaccine elicits higher and more durable NAb titers than the second dose, independent of manufacturer, sex, and age. Our analyses also revealed that vaccinated individuals could be categorized as strong, moderate, and poorly neutralizing responders. After the second dose, 34% of subjects were classified as strong responders, compared to 79% after the third dose. The final months of this study coincided with the emergence of the SARS-CoV-2 Omicron variant and symptomatic breakthrough infections within our study population. Lastly, we show that NAb levels sufficient for protection from symptomatic infection with early SARS-CoV-2 variants were not protective against Omicron infection and disease. This work highlights the need for accessible vaccine response monitoring for use in healthcare, such that individuals, particularly those in vulnerable populations, can make informed vaccination decisions.
Keywords: SARS-CoV-2; lateral flow assay; mRNA vaccines; neutralizing antibody.
Conflict of interest statement
D.F.L. and S.S. are co-founders of Sapphire, the research division of AXIM Biotechnologies. S.S., M.G.M., and A.S.-N. are employed by AXIM. All other authors have no competing interests to declare, and no financial relationships with any organizations that might have an interest in the submitted work in the previous three years.
Figures




Similar articles
-
Boosting of serum neutralizing activity against the Omicron variant among recovered COVID-19 patients by BNT162b2 and CoronaVac vaccines.EBioMedicine. 2022 May;79:103986. doi: 10.1016/j.ebiom.2022.103986. Epub 2022 Apr 8. EBioMedicine. 2022. PMID: 35398786 Free PMC article.
-
Inactivated Vaccines Against SARS-CoV-2: Neutralizing Antibody Titers in Vaccine Recipients.Front Microbiol. 2022 Mar 10;13:816778. doi: 10.3389/fmicb.2022.816778. eCollection 2022. Front Microbiol. 2022. PMID: 35359732 Free PMC article.
-
Assessment of Neutralizing Antibody Response Against SARS-CoV-2 Variants After 2 to 3 Doses of the BNT162b2 mRNA COVID-19 Vaccine.JAMA Netw Open. 2022 May 2;5(5):e2210780. doi: 10.1001/jamanetworkopen.2022.10780. JAMA Netw Open. 2022. PMID: 35532938 Free PMC article.
-
Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine.Front Immunol. 2022 Jan 31;13:827306. doi: 10.3389/fimmu.2022.827306. eCollection 2022. Front Immunol. 2022. PMID: 35173736 Free PMC article.
-
Decreased and Heterogeneous Neutralizing Antibody Responses Against RBD of SARS-CoV-2 Variants After mRNA Vaccination.Front Immunol. 2022 Apr 6;13:816389. doi: 10.3389/fimmu.2022.816389. eCollection 2022. Front Immunol. 2022. PMID: 35464418 Free PMC article.
Cited by
-
Comparative study of anti-SARS-CoV-2 receptor-binding domain total antibody titer before and after heterologous booster with mRNA-based COVID-19 vaccine.Narra J. 2024 Dec;4(3):e788. doi: 10.52225/narra.v4i3.788. Epub 2024 Dec 20. Narra J. 2024. PMID: 39816061 Free PMC article.
-
Tracking Immunity: An Increased Number of COVID-19 Boosters Increases the Longevity of Anti-RBD and Anti-RBD-Neutralizing Antibodies.Vaccines (Basel). 2025 Jan 12;13(1):61. doi: 10.3390/vaccines13010061. Vaccines (Basel). 2025. PMID: 39852840 Free PMC article.
-
Magnitude and Duration of Serum Neutralizing Antibody Titers Induced by a Third mRNA COVID-19 Vaccination against Omicron BA.1 in Older Individuals.Infect Chemother. 2024 Mar;56(1):25-36. doi: 10.3947/ic.2023.0057. Epub 2023 Nov 1. Infect Chemother. 2024. PMID: 38014726 Free PMC article.
-
mRNA-1273 boost after BNT162b2 vaccination generates comparable SARS-CoV-2-specific functional responses in naïve and COVID-19-recovered individuals.Front Immunol. 2023 Apr 21;14:1136029. doi: 10.3389/fimmu.2023.1136029. eCollection 2023. Front Immunol. 2023. PMID: 37153580 Free PMC article.
References
-
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. - DOI - PMC - PubMed
-
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Strålin K., Gorin J.-B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

