NOD1, NOD2, and NLRC5 Receptors in Antiviral and Antimycobacterial Immunity
- PMID: 36146565
- PMCID: PMC9503463
- DOI: 10.3390/vaccines10091487
NOD1, NOD2, and NLRC5 Receptors in Antiviral and Antimycobacterial Immunity
Abstract
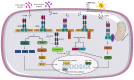
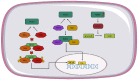
The innate immune system recognizes pathogen-associated molecular motifs through pattern recognition receptors (PRRs) that induce inflammasome assembly in macrophages and trigger signal transduction pathways, thereby leading to the transcription of inflammatory cytokine genes. Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) represent a family of cytosolic PRRs involved in the detection of intracellular pathogens such as mycobacteria or viruses. In this review, we discuss the role of NOD1, NOD2, and NLRC5 receptors in regulating antiviral and antimycobacterial immune responses by providing insight into molecular mechanisms as well as their potential health and disease implications.
Keywords: NLR; antimicrobial immunity; ssRNA virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Activation and regulation mechanisms of NOD-like receptors based on structural biology.Front Immunol. 2022 Sep 15;13:953530. doi: 10.3389/fimmu.2022.953530. eCollection 2022. Front Immunol. 2022. PMID: 36189327 Free PMC article. Review.
-
Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure.J Gen Virol. 2016 Apr;97(4):825-838. doi: 10.1099/jgv.0.000401. Epub 2016 Jan 13. J Gen Virol. 2016. PMID: 26763980 Free PMC article. Review.
-
NOD-Like Receptors: Guards of Cellular Homeostasis Perturbation during Infection.Int J Mol Sci. 2021 Jun 23;22(13):6714. doi: 10.3390/ijms22136714. Int J Mol Sci. 2021. PMID: 34201509 Free PMC article. Review.
-
Nucleotide-binding and oligomerization domain (NOD)-like receptors in teleost fish: Current knowledge and future perspectives.J Fish Dis. 2018 Sep;41(9):1317-1330. doi: 10.1111/jfd.12841. Epub 2018 Jun 29. J Fish Dis. 2018. PMID: 29956838 Review.
-
Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases.Biosci Rep. 2012 Dec;32(6):597-608. doi: 10.1042/BSR20120055. Biosci Rep. 2012. PMID: 22908883 Free PMC article. Review.
Cited by
-
Identification of Mycoplasma pneumoniae proteins interacting with NOD2 and their role in macrophage inflammatory response.Front Microbiol. 2024 May 28;15:1391453. doi: 10.3389/fmicb.2024.1391453. eCollection 2024. Front Microbiol. 2024. PMID: 38863748 Free PMC article.
-
New insights into the structure domain and function of NLR family CARD domain containing 5.Cell Commun Signal. 2025 Jan 23;23(1):42. doi: 10.1186/s12964-024-02012-y. Cell Commun Signal. 2025. PMID: 39849460 Free PMC article. Review.
-
Harnessing Epigenetics: Innovative Approaches in Diagnosing and Combating Viral Acute Respiratory Infections.Pathogens. 2025 Feb 1;14(2):129. doi: 10.3390/pathogens14020129. Pathogens. 2025. PMID: 40005506 Free PMC article. Review.
-
Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity.Cells. 2024 Aug 27;13(17):1437. doi: 10.3390/cells13171437. Cells. 2024. PMID: 39273009 Free PMC article. Review.
-
The Role of Pattern Recognition Receptors in Epigenetic and Metabolic Reprogramming: Insights into Trained Immunity.J Inflamm Res. 2025 Jun 13;18:7795-7811. doi: 10.2147/JIR.S513325. eCollection 2025. J Inflamm Res. 2025. PMID: 40535352 Free PMC article. Review.
References
-
- Fritz J.H., Ferrero R.L., Philpott D.J., Girardin S.E. Nod-like Proteins in Immunity, Inflammation and Disease. Nat. Immunol. 2006;7:1250–1257. - PubMed
-
- Zeromski J., Kierepa A., Boruczkowski M., Kowala-Piaskowska A., Mozer-Lisewska I. The Role Of Nod-Like Receptors In Immunobiology And Medicine. Adv. Cell Biol. 2017;44:201–212.
-
- Macdonald J.A., Wijekoon C.P., Liao K.C., Muruve D.A. Biochemical and Structural Aspects of the ATP-Binding Domain in Inflammasome-Forming Human NLRP Proteins. IUBMB Life. 2013;65:851–862. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

