T-Cell Responses Induced by an Intradermal BNT162b2 mRNA Vaccine Booster Following Primary Vaccination with Inactivated SARS-CoV-2 Vaccine
- PMID: 36146571
- PMCID: PMC9501140
- DOI: 10.3390/vaccines10091494
T-Cell Responses Induced by an Intradermal BNT162b2 mRNA Vaccine Booster Following Primary Vaccination with Inactivated SARS-CoV-2 Vaccine
Abstract
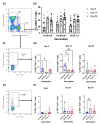
A practical booster vaccine is urgently needed to control the coronavirus disease (COVID-19) pandemic. We have previously reported the safety and immunogenicity of a fractional intradermal booster, using the BNT162b2 mRNA vaccine in healthy volunteers who had completed two doses of inactivated SARS-CoV-2 vaccine. In this study, an intramuscular booster at full dosage was used as a control, and a half-dose vaccination was included for reciprocal comparison. Detailed T-cell studies are essential to understand cellular responses to vaccination. T-cell immunity was examined using S1 peptide restimulation and flow cytometry. The fractional dose (1:5) of the BNT162b2 mRNA vaccine enhanced antigen-specific effector T-cells, but the responses were less remarkable compared to the intramuscular booster at full dosage. However, the intradermal regimen was not inferior to the intramuscular booster a month after boosting. An intradermal booster using only one-fifth of the standard dosage could provide comparable T-cell responses with the fractional intramuscular booster. This work confirms the efficacy of intradermal and fractional vaccination in terms of T-cell immunogenicity in previously immunised populations.
Keywords: COVID-19; T-cells; inactivated SARS-CoV-2; intradermal; mRNA vaccine.
Conflict of interest statement
The authors declare no competing financial or non-financial interests.
Figures

Similar articles
-
Immunogenicity and Safety of an Intradermal BNT162b2 mRNA Vaccine Booster after Two Doses of Inactivated SARS-CoV-2 Vaccine in Healthy Population.Vaccines (Basel). 2021 Nov 23;9(12):1375. doi: 10.3390/vaccines9121375. Vaccines (Basel). 2021. PMID: 34960122 Free PMC article.
-
Reactogenicity and immunogenicity of the intradermal administration of BNT162b2 mRNA vaccine in healthy adults who were primed with an inactivated SARS-CoV-2 vaccine.Vaccine X. 2022 Nov 17;12:100242. doi: 10.1016/j.jvacx.2022.100242. eCollection 2022 Dec. Vaccine X. 2022. PMID: 36415450 Free PMC article.
-
Evaluation of the Safety and Immunogenicity of Fractional Intradermal COVID-19 Vaccines as a Booster: A Pilot Study.Vaccines (Basel). 2022 Sep 8;10(9):1497. doi: 10.3390/vaccines10091497. Vaccines (Basel). 2022. PMID: 36146575 Free PMC article.
-
Analysis of COVID-19 Incidence and Severity Among Adults Vaccinated With 2-Dose mRNA COVID-19 or Inactivated SARS-CoV-2 Vaccines With and Without Boosters in Singapore.JAMA Netw Open. 2022 Aug 1;5(8):e2228900. doi: 10.1001/jamanetworkopen.2022.28900. JAMA Netw Open. 2022. PMID: 36018588 Free PMC article.
-
Immunogenicity and safety of an intradermal ChAdOx1 nCoV-19 boost in a healthy population.NPJ Vaccines. 2022 May 13;7(1):52. doi: 10.1038/s41541-022-00475-z. NPJ Vaccines. 2022. PMID: 35562372 Free PMC article.
Cited by
-
Heterologous COVID-19 Vaccination and Booster with mRNA Vaccine Provide Enhanced Immune Response in Patients with Cirrhosis: A Prospective Observational Study.Vaccines (Basel). 2023 Sep 4;11(9):1455. doi: 10.3390/vaccines11091455. Vaccines (Basel). 2023. PMID: 37766131 Free PMC article.
-
Intradermal Fractional ChAdOx1 nCoV-19 Booster Vaccine Induces Memory T Cells: A Follow-Up Study.Vaccines (Basel). 2024 Jan 23;12(2):109. doi: 10.3390/vaccines12020109. Vaccines (Basel). 2024. PMID: 38400093 Free PMC article.
-
mRNA vaccine designs for optimal adjuvanticity and delivery.RNA Biol. 2024 Jan;21(1):1-27. doi: 10.1080/15476286.2024.2333123. Epub 2024 Mar 26. RNA Biol. 2024. PMID: 38528828 Free PMC article. Review.
-
The characterization of CD8+ T-cell responses in COVID-19.Emerg Microbes Infect. 2024 Dec;13(1):2287118. doi: 10.1080/22221751.2023.2287118. Epub 2024 Jan 11. Emerg Microbes Infect. 2024. PMID: 37990907 Free PMC article. Review.
-
mRNA nanodelivery systems: targeting strategies and administration routes.Biomater Res. 2023 Sep 22;27(1):90. doi: 10.1186/s40824-023-00425-3. Biomater Res. 2023. PMID: 37740246 Free PMC article. Review.
References
-
- World Health Organization Coronavirus Disease (COVID-19) Pandemic. [(accessed on 21 June 2022)]. Available online: https://www.who.int/europe/emergencies/situations/covid-19.
-
- World Health Organization Timeline: WHO’s COVID-19 Response. [(accessed on 21 June 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interact....
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 1 July 2022)]. Available online: https://covid19.who.int/?mapFilter=cases.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

