Evaluation of the Safety and Immunogenicity of Fractional Intradermal COVID-19 Vaccines as a Booster: A Pilot Study
- PMID: 36146575
- PMCID: PMC9505744
- DOI: 10.3390/vaccines10091497
Evaluation of the Safety and Immunogenicity of Fractional Intradermal COVID-19 Vaccines as a Booster: A Pilot Study
Abstract
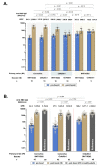
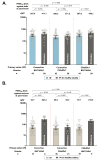
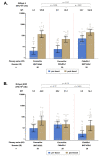
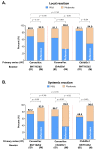
Intradermal vaccination using fractional dosages of the standard vaccine dose is one strategy to improve access to COVID-19 immunization. We conducted a pilot study in healthy adults in Thailand to evaluate the safety and immunogenicity of intradermal administration of fractional doses of ChAdOx1 (1/5th of standard dosage) or BNT162b2 (1/6th of standard dosage) to individuals previously vaccinated (prime) with two-dose intramuscular CoronaVac, ChAdOx1 or BNT162b2. Following an initial immunogenicity exploratory phase for each vaccine combination group (n = 10), a total of 135 participants (n = 45 per group) were recruited to 3 groups (CoronaVac prime-intradermal BNT162b2 boost, CoronaVac prime-intradermal ChAdOx1 boost and ChAdOx1 prime-intradermal BNT162b2 boost) and their immunogenicity data were compared to a previous cohort who received the same vaccine intramuscularly. Two weeks following booster vaccination, neutralizing antibodies against the delta variant were similar between the participants who received intradermal and intramuscular vaccination. However, neutralizing antibodies against the omicron variant in the intradermal BNT162b2 boost groups were ~6-fold lower, while the levels in the ChAdOx1 boost group were similar compared to their respective vaccine regimen given intramuscularly. The intradermal booster significantly increased spike-specific T cell responses in all three groups from pre-booster levels. Local and systemic adverse reactions were milder in intradermal compared to intramuscular injections. Further studies are needed to evaluate the clinical relevance of these findings and the feasibility of administration of intradermal COVID-19 vaccines.
Keywords: COVID-19 vaccination; Thailand; booster; heterologous; intradermal.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this article.
Figures
Similar articles
-
Safety and immunogenicity of intradermal administration of fractional dose CoronaVac®, ChAdOx1 nCoV-19 and BNT162b2 as primary series vaccination.Front Immunol. 2022 Oct 4;13:1010835. doi: 10.3389/fimmu.2022.1010835. eCollection 2022. Front Immunol. 2022. PMID: 36268028 Free PMC article.
-
Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: a randomised, observer-masked, controlled trial in Indonesia.Lancet Infect Dis. 2023 May;23(5):545-555. doi: 10.1016/S1473-3099(22)00800-3. Epub 2023 Jan 11. Lancet Infect Dis. 2023. PMID: 36640798 Clinical Trial.
-
Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study.Lancet. 2022 Feb 5;399(10324):521-529. doi: 10.1016/S0140-6736(22)00094-0. Epub 2022 Jan 21. Lancet. 2022. PMID: 35074136 Free PMC article. Clinical Trial.
-
Safety and immunogenicity against ancestral, Delta and Omicron virus variants following a booster dose of an inactivated whole-virus COVID-19 vaccine (VLA2001): Interim analysis of an open-label extension of the randomized, controlled, phase 3 COV-COMPARE trial.J Infect. 2023 Sep;87(3):242-254. doi: 10.1016/j.jinf.2023.06.022. Epub 2023 Jul 3. J Infect. 2023. PMID: 37406777 Clinical Trial.
-
The Effects of Heterologous Immunization with Prime-Boost COVID-19 Vaccination against SARS-CoV-2.Vaccines (Basel). 2021 Oct 11;9(10):1163. doi: 10.3390/vaccines9101163. Vaccines (Basel). 2021. PMID: 34696271 Free PMC article. Review.
Cited by
-
Immunogenicity and Reactogenicity of Messenger RNA Coronavirus Disease 2019 Vaccine Booster Administered by Intradermal or Intramuscular Route in Thai Older Adults.J Infect Dis. 2023 Oct 3;228(7):868-877. doi: 10.1093/infdis/jiad133. J Infect Dis. 2023. PMID: 37141388 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of intradermal administration of fractional dose CoronaVac®, ChAdOx1 nCoV-19 and BNT162b2 as primary series vaccination.Front Immunol. 2022 Oct 4;13:1010835. doi: 10.3389/fimmu.2022.1010835. eCollection 2022. Front Immunol. 2022. PMID: 36268028 Free PMC article.
-
Is heterologous prime-boost COVID-19 vaccination a concern or an opportunity for Ethiopia?Front Public Health. 2023 Jan 26;10:1046546. doi: 10.3389/fpubh.2022.1046546. eCollection 2022. Front Public Health. 2023. PMID: 36777764 Free PMC article. No abstract available.
-
Immunogenicity, safety and reactogenicity of heterologous (third dose) booster vaccination with a full or fractional dose of two different COVID-19 vaccines: A phase 4, single-blind, randomized controlled trial in adults.Hum Vaccin Immunother. 2023 Aug 1;19(2):2233400. doi: 10.1080/21645515.2023.2233400. Hum Vaccin Immunother. 2023. PMID: 37438960 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of fractional COVID-19 vaccine doses in Nigerian adults: A randomized non-inferiority trial.Sci Rep. 2025 Jul 29;15(1):27614. doi: 10.1038/s41598-025-06536-2. Sci Rep. 2025. PMID: 40730836 Free PMC article. Clinical Trial.
References
-
- The World Health Organization 11 Vaccines Granted Emergency Use Listing (EUL) by WHO. [(accessed on 20 June 2022)]. Available online: https://www.who.int/news/item/19-05-2022-who-validates-11th-vaccine-for-....
-
- Ssentongo P., Ssentongo A.E., Voleti N., Groff D., Sun A., Ba D.M., Nunez J., Parent L.J., Chinchilli V.M., Paules C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022;22:439. doi: 10.1186/s12879-022-07418-y. - DOI - PMC - PubMed
-
- Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., Miller J., Schrag S.J., Verani J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. - DOI - PMC - PubMed
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

