Comparison of the Immune Responses to COVID-19 Vaccines in Bangladeshi Population
- PMID: 36146576
- PMCID: PMC9504987
- DOI: 10.3390/vaccines10091498
Comparison of the Immune Responses to COVID-19 Vaccines in Bangladeshi Population
Abstract
Background: The adaptive immune response is a crucial component of the protective immunity against SARS-CoV-2, generated after infection or vaccination.
Methods: We studied antibody titers, neutralizing antibodies and cellular immune responses to four different COVID-19 vaccines, namely Pfizer-BioNTech, Moderna Spikevax, AstraZeneca and Sinopharm vaccines in the Bangladeshi population (n = 1780).
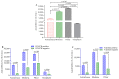
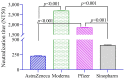
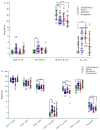
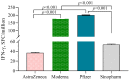
Results: mRNA vaccines Moderna (14,655 ± 11.3) and Pfizer (13,772 ± 11.5) elicited significantly higher anti-Spike (S) antibody titers compared to the Adenovector vaccine AstraZeneca (2443 ± 12.8) and inactivated vaccine Sinopharm (1150 ± 11.2). SARS-CoV-2-specific neutralizing antibodies as well as IFN-γ-secreting lymphocytes were more abundant in Pfizer and Moderna vaccine recipients compared to AstraZeneca and Sinopharm vaccine recipients. Participants previously infected with SARS-CoV-2 exhibited higher post-vaccine immune responses (S-specific and neutralizing antibodies, IFN-γ-secreting cells) compared to uninfected participants. Memory B (BMEM), total CD8+T, CD4+ central memory (CD4+CM) and T-regulatory (TREG) cells were more numerous in AstraZeneca vaccine recipients compared to other vaccine recipients. Plasmablasts, B-regulatory (BREG) and CD4+ effector (CD4+EFF) cells were more numerous in mRNA vaccine recipients.
Conclusions: mRNA vaccines generated a higher antibody response, while a differential cellular response was observed for different vaccine types, suggesting that both cellular and humoral responses are important in immune monitoring of different types of vaccines.
Keywords: B cell; SARS-CoV-2; T cell; cellular immunity; humoral immunity; neutralizing antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Humoral and Cellular Immunogenicity of Six Different Vaccines against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa).Vaccines (Basel). 2022 Jul 27;10(8):1189. doi: 10.3390/vaccines10081189. Vaccines (Basel). 2022. PMID: 35893838 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Comparison of Antibody Levels Produced by Pfizer, AstraZeneca, and Sinopharm Vaccination in COVID-19 Patients in Erbil City-Iraq.Cell Mol Biol (Noisy-le-grand). 2023 Mar 31;69(3):103-112. doi: 10.14715/cmb/2023.69.3.14. Cell Mol Biol (Noisy-le-grand). 2023. PMID: 37300682
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
An Immunological Review of SARS-CoV-2 Infection and Vaccine Serology: Innate and Adaptive Responses to mRNA, Adenovirus, Inactivated and Protein Subunit Vaccines.Vaccines (Basel). 2022 Dec 26;11(1):51. doi: 10.3390/vaccines11010051. Vaccines (Basel). 2022. PMID: 36679897 Free PMC article. Review.
Cited by
-
Antibody response to different COVID-19 vaccines among the migrant workers of Bangladesh.Front Immunol. 2023 Mar 9;14:1128330. doi: 10.3389/fimmu.2023.1128330. eCollection 2023. Front Immunol. 2023. PMID: 36969162 Free PMC article.
-
Serosurveillance among urban slum and non-slum populations immunized with COVID-19 vaccines in Bangladesh.Epidemiol Infect. 2024 Jan 5;152:e14. doi: 10.1017/S0950268823001942. Epidemiol Infect. 2024. PMID: 38178722 Free PMC article.
-
Characteristics and outcomes of SARS-CoV-2 breakthrough infections among double-vaccinated and triple-vaccinated patients with inflammatory rheumatic diseases.RMD Open. 2023 Apr;9(2):e002998. doi: 10.1136/rmdopen-2023-002998. RMD Open. 2023. PMID: 37068915 Free PMC article.
-
Cross-neutralization of Influenza A by SARS-CoV-2 specific neutralizing antibodies and polyclonal plasma: Is pre-exposure to SARS-CoV-2 protective against Influenza A?Heliyon. 2024 Nov 22;10(23):e40638. doi: 10.1016/j.heliyon.2024.e40638. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39654774 Free PMC article.
-
Comparison of SARS-CoV-2 immune responses following vaccination with Comirnaty (Pfizer) and Vaxzevria (AstraZeneca) in healthy individuals with or without prior SARS-CoV-2 infection.Front Immunol. 2025 Jul 17;16:1612288. doi: 10.3389/fimmu.2025.1612288. eCollection 2025. Front Immunol. 2025. PMID: 40746556 Free PMC article.
References
-
- Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020;58:e02107-20. doi: 10.1128/JCM.02107-20. - DOI - PMC - PubMed
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

