Written Briefing and Oral Counseling Increase the Willingness to Receive the SARS-CoV-2 Vaccination among Women in Puerperium: A Qualitative Prospective Cohort Study
- PMID: 36146582
- PMCID: PMC9501465
- DOI: 10.3390/vaccines10091505
Written Briefing and Oral Counseling Increase the Willingness to Receive the SARS-CoV-2 Vaccination among Women in Puerperium: A Qualitative Prospective Cohort Study
Abstract
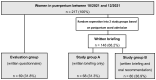
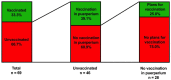
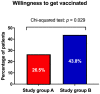
(1) Background: Vaccination rates for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) are low in Austria. International obstetric societies recommend the SARS-CoV-2 mRNA vaccination for women in puerperium. (2) Methods: A prospective two-stage cohort study was conducted at the Medical University of Vienna between October 2022 and December 2022. Firstly, women in puerperium were assigned to the evaluation group (step 1), and secondly, another cohort of unvaccinated women were randomly assigned to study group A (written briefing) or B (written and oral briefing) (step 2). We evaluated the vaccination status among women in the evaluation group and the willingness to receive the vaccination in all three cohorts. (3) Results: We included 217 women in puerperium (evaluation: n = 69, A: n = 68; B: n = 80). In the evaluation group, 66.7% (n = 46/69) of the women were unvaccinated. A total of 45.7% (21/46) of the unvaccinated women categorically declined the SARS-CoV-2 vaccination. A total of 26.5% (n = 18/68) of women in study group A, and 43.8% (n = 35/80) of women in study group B expressed their willingness to receive the vaccination (p = 0.029). There were no differences in willingness to receive the vaccination between different age strata of women in study groups A and B. (D) Conclusion: Our qualitative data demonstrate a benefit from oral counseling in addition to written briefing in order to increase the willingness to receive the vaccination among women in puerperium.
Keywords: SARS-CoV-2; pregnancy; puerperium; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Safety of third SARS-CoV-2 vaccine (booster dose) during pregnancy.Am J Obstet Gynecol MFM. 2022 Jul;4(4):100637. doi: 10.1016/j.ajogmf.2022.100637. Epub 2022 Apr 7. Am J Obstet Gynecol MFM. 2022. PMID: 35398583 Free PMC article.
-
The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Sep 6;22(1):592. doi: 10.1186/s13063-021-05484-2. Trials. 2021. PMID: 34488843 Free PMC article.
-
[Pregnancy, birth, and puerperium with SARS-CoV-2 and COVID-19].Gynakologe. 2020;53(9):614-623. doi: 10.1007/s00129-020-04637-9. Epub 2020 Jul 13. Gynakologe. 2020. PMID: 32836333 Free PMC article. Review. German. No abstract available.
Cited by
-
Risk Awareness as a Key Determinant of Early Vaccine Uptake in the Mpox Vaccination Campaign in an Italian Region: A Cross-Sectional Analysis.Vaccines (Basel). 2023 Nov 27;11(12):1761. doi: 10.3390/vaccines11121761. Vaccines (Basel). 2023. PMID: 38140166 Free PMC article.
-
Interventions to increase vaccination against COVID-19, influenza and pertussis during pregnancy: a systematic review and meta-analysis.J Travel Med. 2023 Dec 28;30(8):taad138. doi: 10.1093/jtm/taad138. J Travel Med. 2023. PMID: 37934788 Free PMC article.
-
The Impact of COVID-19 during Pregnancy on Maternal Hemodynamic Function, Angiogenic Markers and Neonatal Outcome.Viruses. 2024 May 29;16(6):868. doi: 10.3390/v16060868. Viruses. 2024. PMID: 38932160 Free PMC article.
References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

