Novel Synergistic Anti-Enteroviral Drug Combinations
- PMID: 36146673
- PMCID: PMC9505890
- DOI: 10.3390/v14091866
Novel Synergistic Anti-Enteroviral Drug Combinations
Abstract
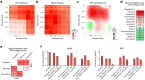
Background: Enterovirus infections affect people around the world, causing a range of illnesses, from mild fevers to severe, potentially fatal conditions. There are no approved treatments for enterovirus infections. Methods: We have tested our library of broad-spectrum antiviral agents (BSAs) against echovirus 1 (EV1) in human adenocarcinoma alveolar basal epithelial A549 cells. We also tested combinations of the most active compounds against EV1 in A549 and human immortalized retinal pigment epithelium RPE cells. Results: We confirmed anti-enteroviral activities of pleconaril, rupintrivir, cycloheximide, vemurafenib, remdesivir, emetine, and anisomycin and identified novel synergistic rupintrivir-vemurafenib, vemurafenib-pleconaril and rupintrivir-pleconaril combinations against EV1 infection. Conclusions: Because rupintrivir, vemurafenib, and pleconaril require lower concentrations to inhibit enterovirus replication in vitro when combined, their cocktails may have fewer side effects in vivo and, therefore, should be further explored in preclinical and clinical trials against EV1 and other enterovirus infections.
Keywords: antiviral drug combination; antiviral strategy; broad-spectrum antiviral agent; echovirus; enterovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Abzug M.J., Cloud G., Bradley J., Sanchez P.J., Romero J., Powell D., Lepow M., Mani C., Capparelli E.V., Blount S., et al. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr. Infect. Dis. J. 2003;22:335–341. doi: 10.1097/01.inf.0000059765.92623.70. - DOI - PubMed
-
- Abzug M.J., Michaels M.G., Wald E., Jacobs R.F., Romero J.R., Sanchez P.J., Wilson G., Krogstad P., Storch G.A., Lawrence R., et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Pleconaril for the Treatment of Neonates with Enterovirus Sepsis. J. Pediatr. Infect. Dis. Soc. 2016;5:53–62. doi: 10.1093/jpids/piv015. - DOI - PMC - PubMed
-
- Hayden F.G., Herrington D.T., Coats T.L., Kim K., Cooper E.C., Villano S.A., Liu S., Hudson S., Pevear D.C., Collett M., et al. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: Results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 2003;36:1523–1532. doi: 10.1086/375069. - DOI - PMC - PubMed
-
- Hayden F.G., Turner R.B., Gwaltney J.M., Chi-Burris K., Gersten M., Hsyu P., Patick A.K., Smith G.J., 3rd, Zalman L.S. Phase II, randomized, double-blind, placebo-controlled studies of ruprintrivir nasal spray 2-percent suspension for prevention and treatment of experimentally induced rhinovirus colds in healthy volunteers. Antimicrob. Agents Chemother. 2003;47:3907–3916. doi: 10.1128/AAC.47.12.3907-3916.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

