CAPG Is Required for Ebola Virus Infection by Controlling Virus Egress from Infected Cells
- PMID: 36146710
- PMCID: PMC9505868
- DOI: 10.3390/v14091903
CAPG Is Required for Ebola Virus Infection by Controlling Virus Egress from Infected Cells
Abstract
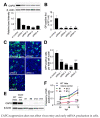
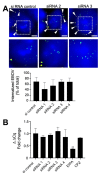
The replication of Ebola virus (EBOV) is dependent upon actin functionality, especially at cell entry through macropinocytosis and at release of virus from cells. Previously, major actin-regulatory factors involved in actin nucleation, such as Rac1 and Arp2/3, were shown important in both steps. However, downstream of nucleation, many other cell factors are needed to control actin dynamics. How these regulate EBOV infection remains largely unclear. Here, we identified the actin-regulating protein, CAPG, as important for EBOV replication. Notably, knockdown of CAPG specifically inhibited viral infectivity and yield of infectious particles. Cell-based mechanistic analysis revealed a requirement of CAPG for virus production from infected cells. Proximity ligation and split-green fluorescent protein reconstitution assays revealed strong association of CAPG with VP40 that was mediated through the S1 domain of CAPG. Overall, CAPG is a novel host factor regulating EBOV infection through connecting actin filament stabilization to viral egress from cells.
Keywords: Ebola virus; actin; assembly; egress; host interaction; trafficking.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Ubiquitin Ligase WWP1 Interacts with Ebola Virus VP40 To Regulate Egress.J Virol. 2017 Sep 27;91(20):e00812-17. doi: 10.1128/JVI.00812-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768865 Free PMC article.
-
ALIX Rescues Budding of a Double PTAP/PPEY L-Domain Deletion Mutant of Ebola VP40: A Role for ALIX in Ebola Virus Egress.J Infect Dis. 2015 Oct 1;212 Suppl 2(Suppl 2):S138-45. doi: 10.1093/infdis/jiu838. Epub 2015 Mar 18. J Infect Dis. 2015. PMID: 25786915 Free PMC article.
-
P300-mediated NEDD4 acetylation drives ebolavirus VP40 egress by enhancing NEDD4 ligase activity.PLoS Pathog. 2021 Jun 10;17(6):e1009616. doi: 10.1371/journal.ppat.1009616. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34111220 Free PMC article.
-
The Role of Exosomal VP40 in Ebola Virus Disease.DNA Cell Biol. 2017 Apr;36(4):243-248. doi: 10.1089/dna.2017.3639. Epub 2017 Feb 8. DNA Cell Biol. 2017. PMID: 28177658 Free PMC article. Review.
-
Host and Viral Proteins Modulating Ebola and Marburg Virus Egress.Viruses. 2019 Jan 3;11(1):25. doi: 10.3390/v11010025. Viruses. 2019. PMID: 30609802 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

