Heterogenous CD8+ T Cell Maturation and 'Polarization' in Acute and Convalescent COVID-19 Patients
- PMID: 36146713
- PMCID: PMC9504186
- DOI: 10.3390/v14091906
Heterogenous CD8+ T Cell Maturation and 'Polarization' in Acute and Convalescent COVID-19 Patients
Abstract
Background: The adaptive antiviral immune response requires interaction between CD8+ T cells, dendritic cells, and Th1 cells for controlling SARS-CoV-2 infection, but the data regarding the role of CD8+ T cells in the acute phase of COVID-19 and post-COVID-19 syndrome are still limited.
Methods: . Peripheral blood samples collected from patients with acute COVID-19 (n = 71), convalescent subjects bearing serum SARS-CoV-2 N-protein-specific IgG antibodies (n = 51), and healthy volunteers with no detectable antibodies to any SARS-CoV-2 proteins (HC, n = 46) were analyzed using 10-color flow cytometry.
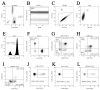
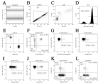
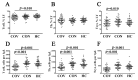
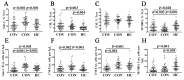
Results: Patients with acute COVID-19 vs. HC and COVID-19 convalescents showed decreased absolute numbers of CD8+ T cells, whereas the frequency of CM and TEMRA CD8+ T cells in acute COVID-19 vs. HC was elevated. COVID-19 convalescents vs. HC had increased naïve and CM cells, whereas TEMRA cells were decreased compared to HC. Cell-surface CD57 was highly expressed by the majority of CD8+ T cells subsets during acute COVID-19, but convalescents had increased CD57 on 'naïve', CM, EM4, and pE1 2-3 months post-symptom onset. CXCR5 expression was altered in acute and convalescent COVID-19 subjects, whereas the frequencies of CXCR3+ and CCR4+ cells were decreased in both patient groups vs. HC. COVID-19 convalescents had increased CCR6-expressing CD8+ T cells. Moreover, CXCR3+CCR6- Tc1 cells were decreased in patients with acute COVID-19 and COVID-19 convalescents, whereas Tc2 and Tc17 levels were increased compared to HC. Finally, IL-27 negatively correlated with the CCR6+ cells in acute COVID-19 patients.
Conclusions: We described an abnormal CD8+ T cell profile in COVID-19 convalescents, which resulted in lower frequencies of effector subsets (TEMRA and Tc1), higher senescent state (upregulated CD57 on 'naïve' and memory cells), and higher frequencies of CD8+ T cell subsets expressing lung tissue and mucosal tissue homing molecules (Tc2, Tc17, and Tc17.1). Thus, our data indicate that COVID-19 can impact the long-term CD8+ T cell immune response.
Keywords: CD3+CD8+; COVID-19; COVID-19 convalescent; IL-27; SARS-CoV-2; Tc1; Tc17; Tc2; chemokine receptors; memory CD8+ T cells; post-COVID-19 syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection.Front Immunol. 2022 Jun 10;13:886431. doi: 10.3389/fimmu.2022.886431. eCollection 2022. Front Immunol. 2022. PMID: 35757700 Free PMC article.
-
A Key Role of CD8+ T Cells in Controlling of Tuberculosis Infection.Diagnostics (Basel). 2023 Sep 15;13(18):2961. doi: 10.3390/diagnostics13182961. Diagnostics (Basel). 2023. PMID: 37761328 Free PMC article.
-
Alterations in B Cell and Follicular T-Helper Cell Subsets in Patients with Acute COVID-19 and COVID-19 Convalescents.Curr Issues Mol Biol. 2021 Dec 30;44(1):194-205. doi: 10.3390/cimb44010014. Curr Issues Mol Biol. 2021. PMID: 35723393 Free PMC article.
-
Chemokine receptors in COVID-19 infection.Int Rev Cell Mol Biol. 2024;388:53-94. doi: 10.1016/bs.ircmb.2024.05.002. Epub 2024 Jun 30. Int Rev Cell Mol Biol. 2024. PMID: 39260938 Review.
-
Sarcoidosis-related autoimmune inflammation in COVID-19 convalescent patients.Front Med (Lausanne). 2023 Dec 21;10:1271198. doi: 10.3389/fmed.2023.1271198. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38179278 Free PMC article. Review.
Cited by
-
Network Analysis for Uncovering the Relationship between Host Response and Clinical Factors to Virus Pathogen: Lessons from SARS-CoV-2.Viruses. 2022 Oct 31;14(11):2422. doi: 10.3390/v14112422. Viruses. 2022. PMID: 36366522 Free PMC article.
-
Persistent CD19+ B cell lymphopenia in critically ill COVID-19 patients 50 days after symptom onset.Front Cell Infect Microbiol. 2024 Nov 22;14:1488607. doi: 10.3389/fcimb.2024.1488607. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39650838 Free PMC article.
-
Deep Immunophenotyping of Circulating T and B Cells in Relapsing Adult-Onset Still's Disease.Curr Issues Mol Biol. 2024 Feb 1;46(2):1177-1191. doi: 10.3390/cimb46020075. Curr Issues Mol Biol. 2024. PMID: 38392193 Free PMC article.
-
Features of Myocarditis: Morphological Differential Diagnosis in Post-COVID-19 Children.Diagnostics (Basel). 2023 Jul 27;13(15):2499. doi: 10.3390/diagnostics13152499. Diagnostics (Basel). 2023. PMID: 37568863 Free PMC article. Review.
-
Recombinant Human Interleukin-2 Corrects NK Cell Phenotype and Functional Activity in Patients with Post-COVID Syndrome.Pharmaceuticals (Basel). 2023 Apr 3;16(4):537. doi: 10.3390/ph16040537. Pharmaceuticals (Basel). 2023. PMID: 37111294 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

