IFIT3 and IFIT5 Play Potential Roles in Innate Immune Response of Porcine Pulmonary Microvascular Endothelial Cells to Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus
- PMID: 36146725
- PMCID: PMC9505468
- DOI: 10.3390/v14091919
IFIT3 and IFIT5 Play Potential Roles in Innate Immune Response of Porcine Pulmonary Microvascular Endothelial Cells to Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus
Abstract
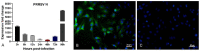
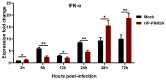
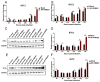
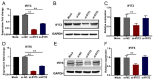
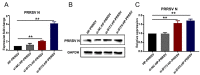
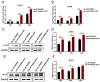
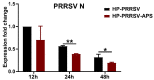
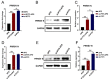
Our previous study has demonstrated that porcine pulmonary microvascular endothelial cells (MVECs) are susceptible to highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV). The innate immune response of MVECs infected with HP-PRRSV would play important roles in controlling virus proliferation, resisting cellular injury, and preventing the virus from spreading to other tissues and organs. Type I interferon is one of the most effective antiviral cytokines in the innate immune response, and interferon-induced proteins with tetratricopeptide repeats (IFITs) are members of interferon-stimulated genes induced by viruses and other pathogens, which are crucial in inhibiting virus proliferation and regulating the innate immune response. However, their effects on HP-PRRSV-induced innate immunity in porcine pulmonary MVECs remain unclear. Here, the roles of IFITs in porcine pulmonary MVECs infected with the HP-PRRSV HN strain were investigated, and the effects of astragalus polysaccharides (APS), a widely used traditional Chinese herbal ingredient with the immunopotentiating effect, on them were studied. The results showed that more autophagosomes were observed in HP-PRRSV-infected MVECs, and the expression of IFN-α, IFIT3, and IFIT5 decreased or increased at different time points after infection. When silencing the genes of IFIT3 or IFIT5, the HP-PRRSV replication in MVECs was significantly increased. The expression of IFIT3 and IFIT5 could be upregulated by APS, whose inhibitory effects on the HP-PRRSV replication significantly declined when the genes of IFIT3 or IFIT5 were silenced. The results suggest that IFIT3 and IFIT5 play an important role in inhibiting the HP-PRRSV replication in porcine pulmonary MVECs, and APS suppress the multiplication of HP-PRRSV by upregulating their expression.
Keywords: HP-PRRSV; MVECs; astragalus polysaccharide; autophagosome; interferon; interferon-inducible protein.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

